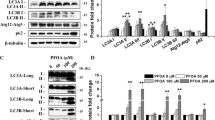
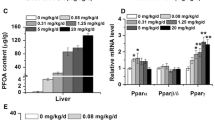
Abstract
Perfluorododecanoic acid (PFDoA) is a ubiquitous environmental pollutant known to cause hepatocellular hypertrophy; however, the mechanisms of hepatotoxicity remain poorly understood. In this study, male rats were exposed to 0, 0.05, 0.2 and 0.5 mg/kg/day of PFDoA for 110 days. After two-dimensional differential gel electrophoresis and MALDI-TOF/TOF analysis, 73 differentially expressed proteins involved in lipid metabolism, inflammation, stress response and other functions were successfully identified. Among them, six significantly changed proteins (CTE1, MTE1, HADHA, ECH1, ALDH2 and CPS1) were found to be regulated by peroxisome proliferator-activated receptor alpha (PPARα). The anti-oxidant enzyme activity assays of superoxide dismutase and glutathione peroxidase and the content of thiobarbituric acid-reactive substances in the liver implied that PFDoA caused oxidative stress. The mRNA levels of PPARα in rat primary hepatocytes were knocked down by lentivirus-mediated RNAi. Furthermore, targeted protein levels of CTE1 and MTE1 were down-regulated, while those of HADHA, ALDH2 and CPS1 were up-regulated. After PFDoA exposure, however, the targeted protein levels of CTE1 and ALDH2 increased compared with those of the knockdown untreated group. The reactive oxygen species (ROS) content in rat hepatocytes assayed by flow cytometry significantly increased in the PPARα knockdown groups, consistent with the PPARα antagonist GW6471- and agonist WY14643-treated groups. These results strongly suggested that PPARα played an important role in suppressing ROS content in hepatocytes following PFDoA exposure.








Similar content being viewed by others
Abbreviations
- CTE1:
-
Cytosolic acyl-CoA thioesterase 1, also known as Acot1 (acyl-CoA thioesterase 1)
- MTE1:
-
Mitochondrial acyl-CoA thioesterase 1, also known as Acot2 (acyl-CoA thioesterase 2)
- HADHA:
-
Hydroxyacyl-coenzyme A dehydrogenase/3-ketoacyl-coenzyme A thiolase/enoyl-coenzyme A hydratase (trifunctional protein), alpha subunit
- ALDH2:
-
Aldehyde dehydrogenase 2 family (mitochondrial)
- ECH1:
-
Enoyl CoA hydratase 1, peroxisomal
- CPS1:
-
Carbamoyl-phosphate synthetase 1
- SOD:
-
Superoxide dismutase
- GPx:
-
Glutathione peroxidase
- TBARS:
-
Thiobarbituric acid-reactive substances
References
Abbott BD, Wolf CJ, Das KP et al (2009) Developmental toxicity of perfluorooctane sulfonate (PFOS) is not dependent on expression of peroxisome proliferator activated receptor-alpha (PPAR alpha) in the mouse. Reprod Toxicol (Elmsford, NY) 27(3–4):258–265. doi:10.1016/j.reprotox.2008.05.061
Alban A, David SO, Bjorkesten L et al (2003) A novel experimental design for comparative two-dimensional gel analysis: two-dimensional difference gel electrophoresis incorporating a pooled internal standard. Proteomics 3(1):36–44
Anderson SP, Yoon L, Richard EB, Dunn CS, Cattley RC, Corton JC (2002) Delayed liver regeneration in peroxisome proliferator-activated receptor-alpha-null mice. Hepatology 36(3):544–554. doi:10.1053/jhep.2002.35276
Bjork JA, Butenhoff JL, Wallace KB (2011) Multiplicity of nuclear receptor activation by PFOA and PFOS in primary human and rodent hepatocytes. Toxicology 288(1–3):8–17. doi:10.1016/j.tox.2011.06.012
Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL (2007) Polyfluoroalkyl chemicals in the US population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602. doi:10.1289/ehp.10598
Chen C, Hennig GE, Whiteley HE, Corton JC, Manautou JE (2000) Peroxisome proliferator-activated receptor alpha-null mice lack resistance to acetaminophen hepatotoxicity following clofibrate exposure. Toxicol Sci 57(2):338–344
Desvergne B, Wahli W (1999) Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 20(5):649–688
DeWitt JC, Shnyra A, Badr MZ et al (2009) Immunotoxicity of perfluorooctanoic acid and perfluorooctane sulfonate and the role of peroxisome proliferator-activated receptor alpha. Crit Rev Toxicol 39(1):76–94
Ding L, Hao F, Shi Z et al (2009) Systems biological responses to chronic perfluorododecanoic acid exposure by integrated metabonomic and transcriptomic studies. J Proteome Res 8(6):2882–2891. doi:10.1021/pr9000256
Dinglasan JA, Bailey M, Park JB, Dhirani AA (2004) Differential conductance switching of planar tunnel junctions mediated by oxidation/reduction of functionally protected ferrocene. J Am Chem Soc 126(20):6491–6497. doi:10.1021/ja0394176
Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL (2012) Hepatocellular hypertrophy and cell proliferation in Sprague–Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARalpha and CAR/PXR. Toxicology 293(1–3):16–29. doi:10.1016/j.tox.2011.12.014
Eriksen KT, Raaschou-Nielsen O, Sorensen M, Roursgaard M, Loft S, Moller P (2010) Genotoxic potential of the perfluorinated chemicals PFOA, PFOS, PFBS, PFNA and PFHxA in human HepG2 cells. Mutat Res 700(1–2):39–43. doi:10.1016/j.mrgentox.2010.04.024
Evans RM, Barish GD, Wang YX (2004) PPARs and the complex journey to obesity. Nat Med 10(4):355–361. doi:10.1038/nm1025
Fujii Y, Yan J, Harada KH et al (2012) Levels and profiles of long-chain perfluorinated carboxylic acids in human breast milk and infant formulas in East Asia. Chemosphere 86(3):315–321. doi:10.1016/j.chemosphere.2011.10.035
Giesy JP, Kannan K (2001) Global distribution of perfluorooctane sulfonate in wildlife. Environ Sci Technol 35(7):1339–1342
Gorrochategui E, Perez-Albaladejo E, Casas J, Lacorte S, Porte C (2014) Perfluorinated chemicals: differential toxicity, inhibition of aromatase activity and alteration of cellular lipids in human placental cells. Toxicol Appl Pharmacol 277(2):124–130. doi:10.1016/j.taap.2014.03.012
Hansen KJ, Clemen LA, Ellefson ME, Johnson HO (2001) Compound-specific, quantitative characterization of organic fluorochemicals in biological matrices. Environ Sci Technol 35(4):766–770
Houde M, Martin JW, Letcher RJ, Solomon KR, Muir DC (2006) Biological monitoring of polyfluoroalkyl substances: a review. Environ Sci Technol 40(11):3463–3473
Houde M, De Silva AO, Muir DC, Letcher RJ (2011) Monitoring of perfluorinated compounds in aquatic biota: an updated review. Environ Sci Technol 45(19):7962–7973. doi:10.1021/es104326w
Hundley SG, Sarrif AM, Kennedy GL (2006) Absorption, distribution, and excretion of ammonium perfluorooctanoate (APFO) after oral administration to various species. Drug Chem Toxicol 29(2):137–145
Hunt MC, Lindquist PJ, Peters JM, Gonzalez FJ, Diczfalusy U, Alexson SE (2000) Involvement of the peroxisome proliferator-activated receptor alpha in regulating long-chain acyl-CoA thioesterases. J Lipid Res 41(5):814–823
Ishii T, Warabi E, Yanagawa T (2012) Novel roles of peroxiredoxins in inflammation, cancer and innate immunity. J Clin Biochem Nutr 50(2):91–105. doi:10.3164/jcbn.11-109
Kersten S, Desvergne B, Wahli W (2000) Roles of PPARs in health and disease. Nature 405(6785):421–424. doi:10.1038/35013000
Kleszczynski K, Gardzielewski P, Mulkiewicz E, Stepnowski P, Skladanowski AC (2007) Analysis of structure–cytotoxicity in vitro relationship (SAR) for perfluorinated carboxylic acids. Toxicol In Vitro 21(6):1206–1211. doi:10.1016/j.tiv.2007.04.020
Kudo N, Suzuki E, Katakura M, Ohmori K, Noshiro R, Kawashima Y (2001) Comparison of the elimination between perfluorinated fatty acids with different carbon chain length in rats. Chem Biol Interact 134(2):203–216
Kudo N, Suzuki-Nakajima E, Mitsumoto A, Kawashima Y (2006) Responses of the liver to perfluorinated fatty acids with different carbon chain length in male and female mice: in relation to induction of hepatomegaly, peroxisomal beta-oxidation and microsomal 1-acylglycerophosphocholine acyltransferase. Biol Pharm Bull 29(9):1952–1957
Lam NH, Cho CR, Lee JS et al (2014) Perfluorinated alkyl substances in water, sediment, plankton and fish from Korean rivers and lakes: a nationwide survey. Sci Total Environ 491–492:154–162. doi:10.1016/j.scitotenv.2014.01.045
Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J (2007) Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci 99(2):366–394. doi:10.1093/toxsci/kfm128
Lin AY, Panchangam SC, Tsai YT, Yu TH (2014) Occurrence of perfluorinated compounds in the aquatic environment as found in science park effluent, river water, rainwater, sediments, and biotissues. Environ Monit Assess 186(5):3265–3275. doi:10.1007/s10661-014-3617-9
Liu C, Yu K, Shi X et al (2007) Induction of oxidative stress and apoptosis by PFOS and PFOA in primary cultured hepatocytes of freshwater tilapia (Oreochromis niloticus). Aquat Toxicol (Amsterdam, Neth) 82(2):135–143. doi:10.1016/j.aquatox.2007.02.006
Liu W, Chen S, Quan X, Jin YH (2008) Toxic effect of serial perfluorosulfonic and perfluorocarboxylic acids on the membrane system of a freshwater alga measured by flow cytometry. Environ Toxicol Chem 27(7):1597–1604. doi:10.1897/07-459
Liu CP, Fu J, Xu FP, Wang XS, Li S (2015) The role of heat shock proteins in oxidative stress damage induced by Se deficiency in chicken livers. Biometals 28(1):163–173. doi:10.1007/s10534-014-9812-x
Mandard S, Muller M, Kersten S (2004) Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci 61(4):393–416. doi:10.1007/s00018-003-3216-3
Martin JW, Mabury SA, Solomon KR, Muir DC (2003) Bioconcentration and tissue distribution of perfluorinated acids in rainbow trout (Oncorhynchus mykiss). Environ Toxicol Chem 22(1):196–204
Martin JW, Smithwick MM, Braune BM, Hoekstra PF, Muir DC, Mabury SA (2004) Identification of long-chain perfluorinated acids in biota from the Canadian Arctic. Environ Sci Technol 38(2):373–380
Mehendale HM (2000) PPAR-alpha: a key to the mechanism of hepatoprotection by clofibrate. Toxicol Sci 57(2):187–190
Minata M, Harada KH, Karrman A et al (2010) Role of peroxisome proliferator-activated receptor-alpha in hepatobiliary injury induced by ammonium perfluorooctanoate in mouse liver. Ind Health 48(1):96–107
Naile JE, Khim JS, Wang T et al (2010) Perfluorinated compounds in water, sediment, soil and biota from estuarine and coastal areas of Korea. Environ Pollut 158(5):1237–1244. doi:10.1016/j.envpol.2010.01.023
Nemali MR, Reddy MK, Usuda N et al (1989) Differential induction and regulation of peroxisomal enzymes: predictive value of peroxisome proliferation in identifying certain nonmutagenic carcinogens. Toxicol Appl Pharmacol 97(1):72–87
Nikitin A, Egorov S, Daraselia N, Mazo I (2003) Pathway studio—the analysis and navigation of molecular networks. Bioinformatics 19(16):2155–2157. doi:10.1093/bioinformatics/btg290
Ogi S, Tanji N, Iseda T, Yokoyama M (1999) Expression of heat shock proteins in developing and degenerating rat testes. Arch Androl 43(3):163–171
Ohmori K, Kudo N, Katayama K, Kawashima Y (2003) Comparison of the toxicokinetics between perfluorocarboxylic acids with different carbon chain length. Toxicology 184(2–3):135–140
Reistad T, Fonnum F, Mariussen E (2013) Perfluoroalkylated compounds induce cell death and formation of reactive oxygen species in cultured cerebellar granule cells. Toxicol Lett 218(1):56–60. doi:10.1016/j.toxlet.2013.01.006
Ren H, Vallanat B, Nelson DM et al (2009) Evidence for the involvement of xenobiotic-responsive nuclear receptors in transcriptional effects upon perfluoroalkyl acid exposure in diverse species. Reprod Toxicol 27(3–4):266–277. doi:10.1016/j.reprotox.2008.12.011
Rich CD, Blaine AC, Hundal L, Higgins CP (2015) Bioaccumulation of perfluoroalkyl acids by earthworms (Eisenia fetida) exposed to contaminated soils. Environ Sci Technol. doi:10.1021/es504152d
Rosen ED, Sarraf P, Troy AE et al (1999) PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell 4(4):611–617
Rosen MB, Abbott BD, Wolf DC et al (2008a) Gene profiling in the livers of wild-type and PPARalpha-null mice exposed to perfluorooctanoic acid. Toxicol Pathol 36(4):592–607. doi:10.1177/0192623308318208
Rosen MB, Lee JS, Ren H et al (2008b) Toxicogenomic dissection of the perfluorooctanoic acid transcript profile in mouse liver: evidence for the involvement of nuclear receptors PPAR alpha and CAR. Toxicol Sci 103(1):46–56. doi:10.1093/toxsci/kfn025
Rosen MB, Schmid JR, Corton JC et al (2010) Gene expression profiling in wild-type and PPARalpha-null mice exposed to perfluorooctane sulfonate reveals PPARalpha-independent effects. PPAR Res 2010. doi:10.1155/2010/794739
Seacat AM, Thomford PJ, Hansen KJ et al (2003) Sub-chronic dietary toxicity of potassium perfluorooctanesulfonate in rats. Toxicology 183(1–3):117–131
Seglen PO (1976) Preparation of isolated rat liver cells. Methods Cell Biol 13:29–83
Senthilkumar K, Ohi E, Sajwan K, Takasuga T, Kannan K (2007) Perfluorinated compounds in river water, river sediment, market fish, and wildlife samples from Japan. Bull Environ Contam Toxicol 79(4):427–431. doi:10.1007/s00128-007-9243-2
Shen L, Hillebrand A, Wang DQ, Liu M (2012) Isolation and primary culture of rat hepatic cells. J Vis Exp (JoVE) (64). doi:10.3791/3917
Shipley JM, Hurst CH, Tanaka SS et al (2004) trans-activation of PPARalpha and induction of PPARalpha target genes by perfluorooctane-based chemicals. Toxicol Sci 80(1):151–160. doi:10.1093/toxsci/kfh130
Takacs ML, Abbott BD (2007) Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol Sci 95(1):108–117. doi:10.1093/toxsci/kfl135
Taniyasu S, Kannan K, So MK et al (2005) Analysis of fluorotelomer alcohols, fluorotelomer acids, and short- and long-chain perfluorinated acids in water and biota. J Chromatogr A 1093(1–2):89–97. doi:10.1016/j.chroma.2005.07.053
Thingholm TE, Larsen MR, Ingrell CR, Kassem M, Jensen ON (2008) TiO2-based phosphoproteomic analysis of the plasma membrane and the effects of phosphatase inhibitor treatment. J Proteome Res 7(8):3304–3313. doi:10.1021/pr800099y
Wetmore BA, Merrick BA (2004) Toxicoproteomics: proteomics applied to toxicology and pathology. Toxicol Pathol 32(6):619–642
Yamada S, Ding Y, Sasaguri Y (2012) Peroxiredoxin 4: critical roles in inflammatory diseases. J UOEH 34(1):27–39
Yang B, Zou W, Hu Z et al (2014) Involvement of oxidative stress and inflammation in liver injury caused by perfluorooctanoic acid exposure in mice. Biomed Res Int 2014:409837. doi:10.1155/2014/409837
Yeldandi AV, Rao MS, Reddy JK (2000) Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat Res 448(2):159–177
Zhang H, Shi Z, Liu Y, Wei Y, Dai J (2008) Lipid homeostasis and oxidative stress in the liver of male rats exposed to perfluorododecanoic acid. Toxicol Appl Pharmacol 227(1):16–25. doi:10.1016/j.taap.2007.09.026
Zhang H, Ding L, Fang X et al (2011) Biological responses to perfluorododecanoic acid exposure in rat kidneys as determined by integrated proteomic and metabonomic studies. PLoS One 6(6):e20862. doi:10.1371/journal.pone.0020862
Zhang W, Liu Y, Zhang H, Dai J (2012) Proteomic analysis of male zebrafish livers chronically exposed to perfluorononanoic acid. Environ Int 42:20–30. doi:10.1016/j.envint.2011.03.002
Acknowledgments
This work was supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB14040202) and the National Natural Science Foundation of China (Grants Nos. 31320103915, 21277143 and 21377128).
Author information
Authors and Affiliations
Corresponding author
Additional information
Hui Liu and Hongxia Zhang have contributed equally to this paper.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Zhang, H., Cui, R. et al. Activation of peroxisome proliferator-activated receptor α ameliorates perfluorododecanoic acid-induced production of reactive oxygen species in rat liver. Arch Toxicol 90, 1383–1397 (2016). https://doi.org/10.1007/s00204-015-1559-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1559-9