Abstract
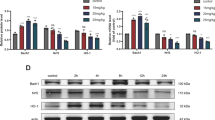
Previous studies have already demonstrated that mitochondria play a key role in Pb-induced apoptosis in primary cultures of rat proximal tubular (rPT) cells. To further clarify the underlying mechanism of Pb-induced mitochondrial apoptosis, this study was designed to investigate the role of mitochondrial permeability transition (MPT) and its regulatory components in Pb-induced apoptosis in rPT cells. Mitochondrial permeability transition pore (MPTP) opening together with disruption of mitochondrial ultrastructure, translocation of cytochrome c from mitochondria to cytoplasm and subsequent caspase-3 activation were observed in this study, suggesting that MPT is involved in Pb-induced apoptosis in rPT cells. Simultaneously, Pb-induced caspase-3 activation and apoptosis can be significantly inhibited by three MPTP inhibitors (CsA, DIDS, BA), which target different regulatory components of MPTP (Cyp-D, VDAC, ANT), respectively, demonstrating that Cyp-D, VDAC and ANT participate in MPTP regulation during lead exposure. Moreover, decreased ATP levels and increased ADP/ATP ratio induced by lead treatment can be significantly reversed by BA, indicating that Pb-mediated ANT dysfunction resulted in ATP depletion. In addition, up-regulation of VDAC-1, ANT-1 together with down-regulation of Cyp-D, VDAC-2 and ANT-2 at both the levels of transcription and translation were revealed in rPT cells under lead exposure conditions. In conclusion, Pb-mediated mitochondrial apoptosis in rPT cells is dependent on MPTP opening. Different expression levels in each isoform of three regulatory components contribute to alteration in their functions, which may promote the MPTP opening.












Similar content being viewed by others
Abbreviations
- MPT:
-
Mitochondrial permeability transition
- rPT:
-
Rat proximal tubular
- MPTP:
-
Mitochondrial permeability transition pore
- CsA:
-
Cyclosporin A
- DIDS:
-
4,4′-Diisothiocyanostilbene-2,2′-disulfonic acid
- BA:
-
Bongkrekic acid
- VDAC:
-
Voltage-dependent anion channel
- ANT:
-
Adenine nucleotide translocase
- Cyp-D:
-
Cyclophilin D
- cyt-c :
-
Cytochrome c
- ROS:
-
Reactive oxygen species
- BCA:
-
Bicinchoninic acid
- ΔΨm :
-
Mitochondrial membrane potential
- PI:
-
Propidium iodide
- CoCl2 :
-
Cobalt chloride
- Calcein-AM:
-
Calcein acetoxymethyl ester
References
Agudo-López A, Miguel BG, Fernández I, Martínez AM (2011) Role of protein kinase C and mitochondrial permeability transition pore in the neuroprotective effect of ceramide in ischemia-induced cell death. FEBS Lett 585:99–103
Armstrong JS (2006) The role of the mitochondrial permeability transition in cell death. Mitochondrion 6:225–234
Baines CP, Kaiser RA, Sheiko T et al (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol 9:550–555
Basso E, Fante L, Fowlkes J et al (2005) Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J Biol Chem 280:18558–18561
Bi J, Wang XB, Chen L et al (2008) Catalpol protects mesencephalic neurons against MPTP induced neurotoxicity via attenuation of mitochondrial dysfunction and MAO-B activity. Toxicol In Vitro 22:1883–1889
Cheng EHY, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301:513–517
Chevrollier A, Loiseau D, Reynier P, Stepien G (2011) Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Bba-Bioenergetics 1807:562–567
De Pinto V, Guarino F, Guarnera A et al (2010) Characterization of human VDAC isoforms: a peculiar function for VDAC3? Bba-Bioenergetics 1797:1268–1275
Dumas JF, Argaud L, Cottet-Rousselle C, Vial G, Gonzalez C, Detaille D, Leverve X, Fontaine E (2009) Effect of transient and permanent permeability transition pore opening on NAD(P)H localization in intact cells. J Biol Chem 284:15117–15125
Godbole A, Varghese J, Sarin A, Mathew MK (2003) VDAC is a conserved element of death pathways in plant and animal systems. Bba-Mol Cell Res 1642:87–96
Goto M, Holgersson J, Kumagai-Braesch M, Korsgren O (2006) The ADP/ATP ratio: a novel predictive assay for quality assessment of isolated pancreatic islets. Am J Transplant 6:2483–2487
Gupta S, Kass GEN, Szegezdi E, Joseph B (2009) The mitochondrial death pathway: a promising therapeutic target in diseases. J Cell Mol Med 13:1004–1033
Heaton MB, Siler-Marsiglio K, Paiva M et al (2013) Ethanol influences on Bax associations with mitochondrial membrane proteins in neonatal rat cerebellum. Dev Neurobiol 73:127–141
Jacotot E, Deniaud A, Borgne-Sanchez A et al (2006) Therapeutic peptides: targeting the mitochondrion to modulate apoptosis. Biochim Biophys Acta 1757:1312–1323
Katoh H, Nishigaki N, Hayashi H (2002) Diazoxide opens the mitochondrial permeability transition pore and alters Ca2+ transients in rat ventricular myocytes. Circulation 105:2666–2671
Keinan N, Pahima H, Ben-Hail D, Shoshan-Barmatz V (2013) The role of calcium in VDAC1 oligomerization and mitochondria-mediated apoptosis. Bba-Mol Cell Res 1833:1745–1754
Lin DT, Lechleiter JD (2002) Mitochondrial targeted cyclophilin D protects cells from cell death by peptidyl prolyl isomerization. J Biol Chem 277:31134–31141
Liu CM, Ma JQ, Sun YZ (2012) Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol Appl Pharmacol 258:330–342
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 25:402–408
Nevin R (2007) Understanding international crime trends: the legacy of preschool lead exposure. Environ Res 104:315–336
Oishi M, Iizumi Y, Taniguchi T et al (2013) Apigenin sensitizes prostate cancer cells to Apo2L/TRAIL by targeting adenine nucleotide translocase-2. PLoS ONE 8:e55922
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Oyagbemi AA, Omobowale TO, Akinrinde AS et al (2014) Lack of reversal of oxidative damage in renal tissues of lead acetate-treated rats. Environ Toxicol. doi:10.1002/tox.21994
Parrish AB, Freel CD, Kornbluth S (2013) Cellular mechanisms controlling caspase activation and function. Cold Spring Harbor Perspect Biol 5(6). doi:10.1101/cshperspect.a008672
Patrick L (2006) Lead toxicity, a review of the literature. Part 1: exposure, evaluation, and treatment. Altern Med Rev 11:2–22
Petronilli V, Miotto G, Canton M, Brini M, Colonna R, Bernardi P, Di Lia F (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734
Ponce-Canchihuamán JC, Pérez-Méndez O, Hernández-Muñoz R et al (2010) Protective effects of Spirulina maxima on hyperlipidemia and oxidative-stress induced by lead acetate in the liver and kidney. Lipids Health Dis 9:35
Shoshan-Barmatz V, Ben-Hail D (2012) VDAC, a multi-functional mitochondrial protein as a pharmacological target. Mitochondrion 12:24–34
Shoshan-Barmatz V, De Pinto V, Zweckstetter M et al (2010) VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol Aspects Med 31:227–285
Sinha K, Das J, Pal PB, Sil PC (2013) Oxidative stress: the mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch Toxicol 87:1157–1180
Trzeciakowski JP, Gardiner L, Parrish AR (2014) Effects of environmental levels of cadmium, lead and mercury on human renal function evaluated by structural equation modeling. Toxicol Lett 228:34–41
Vaseva AV, Marchenko ND, Ji K et al (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149:1536–1548
Wang L, Wang H, Hu M et al (2009) Oxidative stress and apoptotic changes in primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 83:417–427
Xu H, Sun Y, Zhang Y, Wang W, Dan J, Yao J, Chen H, Tian F, Sun X, Guo S, Tian Z, Tian Y (2014) Protoporphyrin IX induces a necrotic cell death in human THP-1 macrophages through activation of reactive oxygen species/c-Jun N-Terminal protein kinase pathway and opening of mitochondrial permeability transition pore. Cell Physiol Biochem 34:1835–1848
Yuan G, Dai S, Yin Z et al (2014) Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol 7:2905–2914
Acknowledgments
This work was supported by the National Nature Science Foundation of China (Nos. 31101870 and 31472251), a foundation for the author of national excellent doctoral dissertation of PR China (No. 201266), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PADD) and the fund of Fok Ying Tung Education Foundation under Grant No. 141022.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Lin Wang and Zong-Ping Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, G., Wang, ZK., Wang, ZY. et al. Mitochondrial permeability transition and its regulatory components are implicated in apoptosis of primary cultures of rat proximal tubular cells exposed to lead. Arch Toxicol 90, 1193–1209 (2016). https://doi.org/10.1007/s00204-015-1547-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-015-1547-0