Abstract.
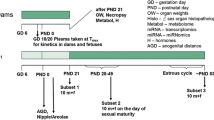
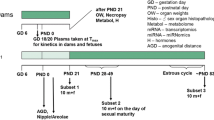
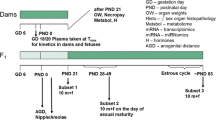
As part of the international validation project to establish the Enhanced OECD Test Guideline 407, we performed a 28-day repeated-dose toxicity study of 17α-methyltestosterone, an exogenous androgen agonist. Special attention was paid to the sensitivity of additional parameters for detecting endocrine-related effects of endocrine-disrupting chemicals, based on the existing Test Guideline 407. Seven-week-old Crj:CD(SD)IGS rats were allocated to one of four groups, each consisting of ten males and ten females, and 17α-methyltestosterone was administered daily by gavage at doses of 0 (control), 5, 20 and 80 mg/kg body weight per day. Male rats were killed on the day after the 28th administration and females on the day of the diestrus stage during the 4 day period after the 28th administration. Male rats receiving 80 mg/kg 17α-methyltestosterone demonstrated decreases in testis and epididymis weights, atrophy of seminiferous tubules and Leydig cells, and degenerated pachytene spermatocytes in the testes and degenerated germ cells in the epididymides as major alterations. Female rats showed abnormal estrous cycles, decreases in ovary and adrenal weights, increase in immature follicles with decreased corpus lutea in the ovaries at doses of 5 mg/kg and higher, as well as atrophy of zona reticularis in the adrenals and increase in mammary gland secretion at 20 mg/kg and above. Dilatation of the lumina and apoptosis of endometrial cells in the uterus, mucinification in the vagina and increase in serum follicle-stimulating hormone were seen with 80 mg/kg. Among the parameters examined in the present experimental system, effects of 17α-methyltestosterone on endocrine-related organs were detected in organ weights and histopathological examination of both sexes, and in serum hormones and estrous cycle of females. Based on these results, the no-observed-adverse-effect level (NOAEL) in the present study was estimated to be below 5 mg/kg per day. In particular, effects were most sensitively detected by organ weights and histopathological examination of sexual organs.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Okazaki, K., Imazawa, T., Nakamura, H. et al. A repeated 28-day oral dose toxicity study of 17α-methyltestosterone in rats, based on the 'Enhanced OECD Test Guideline 407' for screening the endocrine-disrupting chemicals. Arch Toxicol 75, 635–642 (2002). https://doi.org/10.1007/s00204-001-0292-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-001-0292-8