Abstract
A tannase-positive Bacillus gottheilii M2S2 and Bacillus cereus M1GT were co-cultivated for the production of gallic acid using tannic acid as the sole carbon source through submerged fermentation. Taguchi orthogonal array of design of experimental methodology was used to estimate the influence and significance of tannic acid concentration, glucose concentration, agitation speed, and inoculum size on the gallic acid production in a shake flask. Among all the factors, agitation speed contributed the highest for gallic acid production (28.28%), followed by glucose concentration (21.59%), inoculum size (19.6%), tannic acid concentration (19.54%), and pH (11.09%). Validation experiments were executed at the found optimized conditions which resulted in a 6.36-fold increase in gallic acid yield compared to unoptimized conditions. Further, the kinetics of growth, tannic acid degradation, and gallic acid yield were evaluated at the optimized conditions. The kinetic parameters Y x/s, Y p/s, and Y p/x were determined as 0.292 mg of cells/mg of tannic acid, 22.2 µg of gallic acid/mg of tannic acid, and 70.76 µg of gallic acid/mg of cells with a growth rate of 0.273 h −1 after 24 h of fermentation. Finally, the antimicrobial activity of the product gallic acid was investigated against food-borne pathogenic E. coli, S. aureus, and Serriatia marcescens and showed a zone of inhibition of 2 cm, 1.6 cm, and 1.3 cm, respectively, using the agar disc diffusion technique. Thus, the cost-effective bioproduct gallic acid proved to be potentially effective to control food poisoning diseases and preserve foodstuff.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
3,4,5-trihydroxy benzoic acid is an organic compound commonly known as Gallic acid, discovered by Carl Wilhelm Scheele in 1878. It is an integral compound of tannic acid molecules found in plants and fruits (Aguilar-Zarate et al. 2015; Chandrasekaran and Beena, 2013). Gallic acid devises wide applications in the synthesis of an antibacterial drug called trimethoprim (pharmaceutical industry); in the synthesis of pyrogallol, inks, dihydroxyacetone, alkaloids, and photographic developer (chemical industry); and in the production of an antioxidant, propyl gallate, and gallate esters, as preservatives (food industry) (Kanpiengjai et al. 2020; Banerjee et al. 2007). It is also known to exhibit several pharmacological properties like antibacterial, antiallergic, antioxidant, antimutagenic, anti-inflammatory, neuroprotective, and anticarcinogenic activities (Kanpiengjai et al. 2020; Verma et al. 2013; Bajpai and Patil 2008; Kroes et al. 1992). Gallic acid being a phenolic component showed delayed antimicrobial resistance against pathogens of bovine respiratory diseases (Rajamanickam et al. 2019). Gallic acid also causes damage to the sensitive strains by penetrating the cell membranes and chelating with the calcium ions which results in the death of pathogens (Sarjit et al., 2015), which represents that gallic acid is a potent antimicrobial product. The global yearly demand for gallic acid is about 8000 tonnes, 75% of it is used in the production of trimethoprim (Chandrasekaran and Beena, 2013; Lokeswari, 2010a), also the natural existence is limited.
In India, gallic acid is manufactured by extracting tannic acid from imported tara powder and acid hydrolyzing it with sulfuric acid at high temperatures to obtain gallic acid which is crystallized to obtain the final product trimethoprim (an antibacterial agent). The manufacture of trimethoprim involves three stages, viz;
While most of the manufacturers of trimethoprim are having the technology of stage III, stages I and II are not very much established. Indian companies have, by and large, been successful in achieving good efficiencies. Even though the production process is somewhat simple and their production units have been stabilized, the important raw material tara powder is expensive as it is presently imported and also has irregular supply demanding an alternate source. The major constituent of tara powder is tannic acid about 40%. Conventionally, the compound 3,4,5 trimethoxy benzaldehyde is produced by chemical methods, but these processes generate not only the environment but also processing problems like low purity, high cost, and low yield. Therefore, this gallic acid for the synthesis of drug intermediates stands unsuitable. On the other hand, microbial tannase can be used to hydrolysis tannins into gallic acid which is a simpler method, requires fewer stages, and is a non-polluting process (Chandrasekaran and Beena, 2013; Lokeswari 2010a; Lokeswari et al. 2010b). The tannase is an inducible enzyme secreted by microbes, plants, and animals, upon hydrolysis of tannic acid, liberates gallic acid and glucose in the ratio of 9:1. Among them, microbial tannase mainly from fungi is well reported (Arshad et al. 2019; Cruz et al. 2017; Xiao et al. 2015; Mondal et al. 2001). Still, the documents on the degradation of tannins are scarce. Predominantly used strain for the hydrolysis of tannic acid into gallic acid was Aspergilli (Saeed et al. 2020; Arshad et al. 2019; Lokeswari 2010a; Bajpai and Patil, 2008; Banerjee et al. 2007), among bacteria Enterobacter sp. (Sharma et al. 2017), Bacillus subtilis AM1 (Aguilar-Zarate et al. 2015), Lactobacillus plantarum CIR1 (Aguilar-Zarate et al. 2015), Corynebacterium sp. (Deschamps and Lebeault, 1984), Klebsiella pneumonia (Deschamps and Lebeault, 1984), etc. have been reported to yield gallic acid.
The gallic acid and tannase can be co-produced using both submerged fermentation (SmF) and solid state fermentation (SSF) processes (Belur and Mugeraya, 2011). Yet, submerged fermentation is the most ideal choice for bacterial growth than SSF as they need high moisture content, doubling time is short, purification of the product is easier, and control of the fermentation is simpler (Belur and Mugeraya, 2011). Upon the unearthing of an enzyme tannase in 1867, excessive research was carried out for its products mainly from fungus, as tannins were considered bacteriostatic. In the year 1983, the very first finding of bacterial tannase was reported (Deschamps et al. 1983) and the strong focus on bacterial tannase and gallic acid synthesis was seen from 1990 onwards (Mondal et al. 2001; Das Mohapatra et al. 2006; Selwal et al. 2010; Natarajan and Rajendran, 2012). In a natural habitat, fermentations generally occur in mixed cultures. The mixed culture may show interactions among themselves and exhibit kinetic reactions (Purohit et al. 2006). These interactions of the microbial population also depend on the environmental factors and create two types of association, synergistic (proliferates the microorganisms and increases the metabolite or enzyme production) and antagonistic (inhibits the growth of other microorganisms) (Abdullah et al. 2018). Bacterial co-culturing is a unique method in which two or more different cultures develop under the same environmental conditions with some synergy among them. In this study, two different bacterial strains with a positive synergy were used to improve the metabolite gallic acid and enzyme tannase synthesis rather than bacterial monoculture. There are quite a few articles reported on bacterial co-culture to enhance enzyme productions like alpha-amylase (Abdullah et al. 2018; Pandey et al. 1999). Though, this is the first report on the production of gallic acid from bacterial strains using the co-culturing method.
In this communication, we made attempts to enhance gallic acid production by co-culturing Bacillus gottheilii M2S2 and Bacillus cereus M1GT through SmF. Taguchi orthogonal array of design of experimental technique was used to study the effects of process parameters, including tannic acid concentration, glucose concentration, agitation speed, inoculum size, and initial pH. Besides, to understand the design of fermentation processes utilizing the co-culturing method, the kinetic constants µ (specific growth rate), Xm (maximum biomass concentration), Yx/s (biomass yield coefficient based on substrate consumption), Yp/s (product yield coefficient based on substrate consumption), and Yp/x (product yield coefficient based on biomass) were evaluated at the optimized process conditions. Finally, the product gallic acid produced was used to test the antimicrobial activity against the bacterial strains causing food poisoning diseases.
Materials and methods
Microorganism and bacterial co-culture cell suspension preparation
Gallic acid-producing strains of Bacillus gottheilii M2S2 and Bacillus cereus M1GT were previously isolated from tannery effluent soil and gastrointestinal tract of goat, respectively, by adopting liquid enrichment and spread plate methods as described elsewhere (Subbalaxmi and Murty, 2016; Selvaraj et al. 2021). The ribosomal RNA gene sequence of newly isolated Bacillus gottheilii M2S2 and Bacillus cereus M1GT was deposited at Gen Bank bearing an accession ID as KU866380 and KX033490, respectively.
A one loop-full of strain Bacillus gottheilii M2S2 or Bacillus cereus M1GT was grown in 10 mL of sterilized nutrient broth for 20 h at 180 rpm and 32 °C. Then, 1 mL of the respective strain was further grown in 50 mL sterilized nutrient broth under the same condition. Further to obtain co-culture suspension, the individual suspensions of Bacillus gottheilii M2S2 and Bacillus cereus M1GT were mixed in equal proportions of volume ratio of 1:1 as these strains exhibited approximately equal proportions of cell quantity based on the viable cell count experiment (Li et al. 2018).
Co-culture fermentation
To run the co-culture submerged fermentation, 2 mL of 20 h old Bacillus gottheilii M2S2 inoculum and 2 mL of 20 h old Bacillus cereus M1GT inoculum each containing 4 × 1011 CFU/mL were aseptically mixed into 250 mL Erlenmeyer flask containing 100 mL of production media composed of 1% (w/v) of tannic acid (as carbon source and tannase inducer), 0.5% (w/v) of NH4NO3 (as nitrogen source), and supplemented with 0.05% (w/v) of K2HPO4, 0.05% (w/v) of KH2PO4, 0.05% (w/v) of MgSO4.7H2O, 0.05% (w/v) of NaCl; adjusted the pH to 5.0. The Erlenmeyer flasks were incubated in a rotary shaker at 32 °C and 180 rpm for 24 h (Purohit et al. 2006; Banerjee et al. 2005). Experiments were carried out in triplicates and after fermentation, the gallic acid content, tannic acid content, biomass, and pH were determined.
Optimization of gallic acid production using Taguchi’s L16 orthogonal array design
Based on our preliminary studies using co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT, process parameters such as tannic acid concentration, glucose concentration, agitation speed, initial pH, and inoculum size were found to be the most influential parameters for gallic acid production. Since the variations in these parameters can alter the course of an experiment, in turn, the “response parameter”, i.e., gallic acid concentration can be increased, thereby helping to set the experimental parameters while designing an experiment.
To study the effect of both chemical and physical parameters on gallic acid production by bacterial co-culture and to enhance its concentration for maximum gallic acid production an ordered L16 orthogonal array of experiments was used. The above-mentioned five parameters were evaluated and optimized at four different levels designated by L1, L2, L3, and L4 as with 16 experimental runs as depicted in Table 1. Concerning the optimization of process conditions, each column in Table 1 would represent specific process parameters, and each row would depict experimental runs with different combinations of parameters. The main aim of the optimization of process conditions was to enhance gallic acid production. For this, Taguchi’s statistical method was used to find the optimal conditions which are based on the signal-to-noise ratio (S/N) function. This method eliminates the experimental variations improving the overall outcome of the experiment. This is done by setting the ‘signal-to-noise’ ratio as “larger the better” (Singh and Verma, 2019; Mohan and Reddy, 2013). The main aim of this statistical method is to find the optimal experimental conditions to enhance the yield of gallic acid by using the aforementioned option. Here, the gallic acid concentration is measured as a response, and the formula for the S/N ratio is given:
where S/N = signal-to-noise ratio; N = number of experimental runs; and Xi = gallic acid concentration of respective runs. The optimal values of each parameter are those at the largest S/N ratio.
The design matrix, its analysis, and the process of experimental runs were evaluated using statistical software MINITAB 17 (Trial version). The evaluated data were taken to rank the most influential parameters on the yield of gallic acid and to find the best fermentation environments for co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT to produce the highest gallic acid. To validate Taguchi’s L16 orthogonal array design, the experiments were carried out in triplicates at determining optimized process conditions.
Kinetic studies of gallic acid production at optimized process conditions
The kinetics of gallic acid production from co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT was evaluated in optimized conditions, which was found based on the Taguchi’s L16 orthogonal array approach as described below. The composition of fermentation media and cultural conditions are as follows: tannic acid 1% (w/v), agitation speed 120 rpm, and inoculum size 10% (v/v); whereas other components were maintained at 0.5% (w/v) of NH4NO3, 0.05% (w/v) of K2HPO4, 0.05% (w/v) of KH2PO4, 0.05% (w/v) of MgSO4.7H2O, and 0.05% (w/v) of NaCl; at optimum pH 6.0. Then, for every 4 h of fermentation, tannic acid content, gallic acid, biomass concentration, total protein, and glucose were determined. Experiments were carried out independently in triplicates.
To better understand the bacterial co-culture of gallic acid production, different kinetic parameters, i.e., specific growth rate (µ), biomass yield coefficient based on substrate utilization (Yxs), product yield coefficient based on biomass (Ypx), product yield coefficient based on substrate utilization (Yps), the specific rate of substrate utilization (qs), and specific rate of product formation (qp) were estimated based on the methods described by Doran (1995). The following are the set of equations used to estimate the fermentation kinetic parameters:
In the above equations, µ, X, S, P, and t are specific growth rate, biomass concentration, substrate (tannic acid) concentration, product (gallic acid) concentration, and the fermentation time, respectively. All these fermentation kinetic parameters were studied in the batch process mode at optimized environmental conditions.
Analytical methods
After submerged fermentation, the total fermentation broth was subjected to centrifugation (10,000 rpm, 15 min, and 4 °C). The cell-free extract was utilized for analysis of gallic acid, tannic acid, and final pH; whereas wet residue (pellet) was used to determine dry cell weight.
The product gallic acid was estimated spectrophotometrically using rhodanine as a coloring agent and methyl gallate as a substrate (Sharma et al. 2000). The amount of gallic acid released was correlated with the gallic acid standard curve, which was attained by measuring the absorbance of different concentrations of standard gallic acid solutions ranging from 0 to 100 nmol.
Further, the protein precipitation method of Ann-Hagerman and Larry-Butler (1978) was adopted to quantify the tannic acid content in the cell-free extract.
Finally, the dry cell weight method was used to determine the biomass concentration; the pellet obtained after centrifugation of fermentation broth was dried at 80 °C in a hot air oven until it reaches constant weight. The biomass concentration was calculated as defined below:
Antibacterial activity of the gallic acid
Bacterial strains
The effectiveness of antibacterial activity of gallic acid produced by co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT under SmF was estimated using two strains of Gram-positive (Bacillus species and Staphylococcus) and two strains of Gram-negative (Escherichia coli and Serratia marcescens) bacteria causing food poisoning disease. The above-mentioned strains for antibacterial check are procured from the culture collection of the Institute of Microbial Technology, Chandigarh, India.
Inoculum preparations
The above-mentioned bacterial strains were grown overnight in Nutrient agar slants at 32 °C. Further, the growth of bacterial strains was harvested and diluted using sterile saline water to obtain a viable cell count of 106 CFU/mL and its absorbance was adjusted at 600 nm using a spectrophotometer.
Antibacterial activity of gallic acid produced
The diffusion assay method was adopted to estimate the antimicrobial activity of the gallic acid produced under SmF by co-culture fermentation of Bacillus gottheilii M2S2 and Bacillus cereus M1GT. The gallic acid extract was steam-sterilized at 121 °C and 15 psi for 15 min. The overnight grown bacterial suspension of 0.1 mL was spread plated on petri dishes containing 20 mL solidified nutrient agar medium. Further, the sterilized cork borer holes were punched on nutrient agar plates. Then, the gallic acid solution of 0.5 mL was poured into the well and the plates were kept in the incubator for 24 h at 37 °C. The exhibition of clear zones was recorded by the Vernier caliper and considered as the presence of antibacterial activity.
Results and discussion
Microbial production of metabolites mainly depends on the physical and chemical environment of the microorganism employed. Initially, the production of gallic acid was studied by co-culture fermentation of Bacillus gottheilii M2S2 and Bacillus cereus M1GT at 32 °C, 180 rpm and for 48 h in production medium (% w/v) comprising tannic acid, 1; NH4NO3, 0.5; K2HPO4, 0.05; KH2PO4, 0.05; MgSO4. 7H2O, 0.05; and NaCl, 0.05; with pH of 5.0. The maximum gallic acid concentration of 22.76 µg/mL was exhibited at 24 h of fermentation. The product gallic acid was found to be growth associated and also similar results have been observed in Lactobacillus plantarum (Natarajan et al. 2011) and Bacillus sphericus (Raghuwanshi et al. 2011). Based on the preliminary studies, the parameters such as tannic acid (carbon source), glucose (inducer), inoculum size, initial pH, and agitation speed were found to be most critical for the production of gallic acid by co-culture fermentation of Bacillus gottheilii M2S2 and Bacillus cereus M1GT.
Yet, the interaction effects of these parameters on the gallic acid yield were not possible with traditional methods of optimization, which has proven to exhibit a significant effect on the regulation of metabolism (Subbalaxmi and Murty, 2016). Hence, optimization of fermentation media was examined for gallic acid yield with five critical parameters at their selected levels as shown in Table 1.
Regression analysis
The co-culturing of Bacillus gottheilii M2S2 and Bacillus cereus M1GT was adopted in this study and the strains have shown a good yield of gallic acid in the initial study completed. As the bacterial co-culture production of gallic acid was not reported, hence this study aimed to check the prospect of the bacterial cultures producing a high amount of gallic acid on a laboratory scale. The process parameters such as tannic acid concentration, glucose concentration, agitation speed, initial pH, and inoculum size were considered in the development of mathematical models. Table 1 illustrates the final gallic acid concentration at different levels of fermentation media compositions. The Taguchi L16 design matrix showed significant differences in the gallic acid yield (Table 1). Among all 16 experimental trials, the maximum and minimum gallic acid concentration was achieved in Run 5 (143.52 µg/mL: Tannic acid, 1% w/v; Glucose, 0% w/v; Agitation speed, 120 rpm; pH, 5 and inoculum size, 10% v/v) and Run 16 (19.79 µg/mL: Tannic acid, 2% w/v; Glucose, 0.4% w/v; Agitation speed, 100 rpm; pH, 6 and inoculum size, 6% v/v), respectively, at 32 °C and 24 h of fermentation. The differences in the concentrations of gallic acid with a change in the media composition indicate that the production of fermentation products is the main function of media composition. This statistical method of optimization showed a significant increase in the gallic acid yield from 22.76 to 143.52 µg/mL when compared to the traditional method of media optimization.
The S/N ratio in Taguchi design is generally used to eliminate the experimental variations caused due to uncontrollable parameters. Table 1 depicts the results of the L16 orthogonal array for the production of gallic acid, and the S/N ratio (larger is better). It is an important parameter in Taguchi’s L16 orthogonal array design to identify the optimal conditions for the process. The values of the S/N ratio tell which combination of parameters has the maximum effect on the response, i.e., gallic acid concentration. The upper value of the S/N ratio indicates that those constituents in composition have the maximum effect on the gallic acid yield. In this study, based on the main effects of each parameter the order of parameters ranked as agitation speed > glucose concentration > inoculum size > tannic acid concentration > initial pH, indicating that agitation speed had the highest effect and initial pH exhibited the least effect on the gallic acid yield by co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT. The main effect plots of individual parameters are generated by plotting the response average against each parameter level using the software MINITAB (Fig. 1). These plots explain how an individual parameter affects the response, i.e., gallic acid yield. The main effect of a parameter can be negligible or zero if the line is horizontal to the X-axis. If the line makes a larger deviation in vertical location from the horizontal X-axis, then the main effect can be maximum. In this study, it is noticed that tannic acid at level 2 (1% w/v), glucose at level 1 (0% w/v), agitation speed at level 2 (120 rpm), initial pH at level 4 (7.0), and inoculum size at level 3 (8% v/v) exhibited the maximum main effect on the gallic acid yield (Fig. 1). These levels and their values indicate the optimal process conditions for the growth and metabolism of Bacillus gottheilii M2S2 and Bacillus cereus M1GT. The optimal process conditions can also be predicted from the response table (Table 1).
The response table for the S/N ratio for each parameter and each parameter is ranked based on the delta, which generally relates to the relative degree of the effects of different parameters (Table S1). A parameter with the highest delta is ranked 1. A parameter with a high S/N ratio indicates the better performance of that parameter for the production of gallic acid with the least error of measurement. The graphical representation of the S/N ratio is provided in Figure S1. Agitation speed exhibits the maximum contribution and is ranked 1 for delta followed by inoculum size and concentration of glucose as shown in Table S1. The mean in the Taguchi design represents the average response of gallic acid yield for each combination of parameters and levels. The maximum percent of this contributes greatly to enhancing gallic acid yield. Based on the mean response as shown in Table S1, agitation speed showed maximum influence followed by glucose content, inoculum size, tannic acid concentration, and initial pH.
During the analysis of the effect of each parameter with their levels, the observation was noticed that the highest average effect was seen at the agitation speed of level 2. Subsequently, the next highest were glucose concentration, inoculum size, tannic acid concentration, and initial medium pH at levels 1, 3, 2 and 4, respectively (Fig. 1 and Table S2). The effect of the parameter at the individual level is defined as the difference between the average value at a high and low level of each parameter. Larger the difference, the stronger the significance. The positive or negative signs of values of the effect of different parameters at each level will determine whether the contribution towards gallic acid production has increased or decreased (Table S2 and Table S3). It was found that the relative influence of process parameters on gallic acid is shown below for the fermentation period of 24 h at 32 °C.
Agitation speed > Glucose concentration > Inoculum size > Tannic acid concentration > Initial pH.
Impact of interaction effects of parameters
The MINITAB 17.0 software generated the interaction effects and was examined individually to better understand the production of gallic acid by co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT. The severity index (SI) helps to understand the interaction of two parameters on gallic acid yield at defined levels. The Severity Index measures the angle between the two straight interaction lines in an interaction plot. SI also indicates the strength of the presence of interaction. The angle between the interaction lines can vary between 0 and 90 degrees and is expressed on a scale of 0–100 as the SI (Table 2). The results of predicted interactions shown in Table 2 are in good agreement with the interaction plots shown in Figures S2, S3, and S4.
Based on the statistical results represented in Table 2, it can be noticed that the interaction of initial pH and glucose concentration of the media showed the highest effect of 96.81%. But, it is also intrusive to note that the initial pH is having the least percent factor of 11.09% (Table 3) which showed maximum interaction with the parameter glucose concentration (21.59%). Among all the selected parameters, the interaction between glucose concentration and agitation speed exhibited the least severity index of 23.46%. Hence from the interaction studies, it can be established that the effect of individual parameters on gallic acid is different and also in the grouping is independent of the effect of the individual parameter. This result suggests that the insignificant parameters at their levels can be very much significant when interacting with other parameters to enhance the gallic acid yield.
Analysis of variance
The results of Taguchi’s design matrix were analyzed and evaluated the contribution of each parameter toward the yield of gallic acid by Analysis of variance (ANOVA). Further, the quality of the experimental results was given by F ratio. This is a test statistic used for more than a few independent parameters. The test statistics can be calculated as follows:
If the sum of squares (SS) measures the variance, then the test statistic can be shown as
And the total variance is calculated as, \({SS}_{Total}= {SS}_{Model}+ {SS}_{Residual,}\)where SSModel, expected variance; and SSResidual, random variance.
F ratio and a Mean sum of squares (MS) can be calculated using the formula shown below:
ANOVA was carried out to evaluate the variation in gallic acid production caused due to each parameter and also to estimate the optimal level value of a parameter for the maximum product formation. The ANOVA exhibited the values of the model sum of squares, mean squares, and Variance as 15,988, 3198, and 1066, respectively (Table 3). The model obtained from ANOVA for gallic acid production showed multiple regression coefficients (R2) of 0.998, which indicates that the model can explain a 99.8% variation in the gallic acid yield.
Based on the statistical calculations and predictive analysis, the optimized values of the individual parameter are obtained and depicted in Table 4. The statistical analysis of Taguchi’s L16 experimental design data for gallic acid yield revealed that agitation speed contributed the maximum effect of 24.32% and initial pH exhibited the least effect of 6.03% on gallic acid production at optimum conditions.
The final optimum media composition (% w/v) for an increased gallic acid yield by co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT is, tannic acid, 1; glucose, 0; agitation speed, 120 rpm; and inoculum size, 10% (v/v); whereas, other components were maintained (% w/v) at 0.5, NH4NO3; 0.05, K2HPO4; 0.05, KH2PO4; 0.05, MgSO4.7H2O; 0.05, NaCl; and optimum pH 6.0 (Table 4).
To validate the results of the experimental design matrix, trials were carried out in triplicates under the optimized conditions and observed the gallic acid yield of 578.26 µg/mL which is in good agreement with the MINITAB software predicted value of 574.08 µg/mL. This optimum condition produced 578.26 µg/mL of gallic acid concentration in 24 h of fermentation, whereas 91.04 µg/mL was observed before process optimization and hence the yield was enhanced by 6.35-fold by Taguchi’s L16 orthogonal array of experimental design.
Kinetic studies of gallic acid production at optimized process conditions
The kinetic results of the co-culture of Bacillus gottheili M2S2 and Bacillus cereus M1GT in shake flasks for gallic acid yield are shown in Fig. 2. Biomass concentration was inadequate during the initial 4 h of inoculation; this condition is most common during growth where the lag period is vital to acclimatize to the new environment during this period the. During the lag period, the growth was amplified from 0.07 g/L to 0.16 g/L whereas tannin content declined from 9.4 g/L to 9.1 g/L. The lag phase is followed by the log phase, in which the tannic acid content is reduced to 2.28 g/L from 9.1 g/L; whereas, biomass growth was raised to 4.18 g/L from 0.16 g/L. During the log phase, biosynthesis and excretion of gallic acid were initiated, and by the end of the growth phase after 24 h of fermentation, the maximum increase was observed to be 586 µg/mL. Relatively constant biomass and a decrease in gallic acid production were observed in the stationary phase, continuing until the death phase. By these findings, it was demonstrated that the gallic acid yield was growth associated and that both strains of bacteria were rapidly growing strains with tremendous adaptability to the fermentation medium.
During the fermentation process, biomass and product yield factors were estimated (Table 5). After 24 h of fermentation, it was found that maximum Yp/s and Yp/x were 175.95 mg/g of tannic acid and 172.89 mg/g of biomass, respectively. This indicates that gallic acid production followed the growth pattern of Bacillus gottheilii M2S2 and Bacillus cereus M1GT, indicating that gallic acid was the primary metabolite produced. On further analysis, it was observed 4 h after fermentation that the maximum cell growth rate was 0.701 h−1. This demonstrates that both strains grew much faster during the initial exponential phase. For the production of Tannase in SSF under optimized conditions, reported biomass, product yield, and specific growth rate were 0.276 g/g, 0.177 U/g, and 0.0703 h−1, respectively (Selvaraj and Vytla, 2017). The evaluation of the specific rate of product formation (qp), together with maximum biomass concentration (Xm) helps in distinguishing whether the increased production rate was due to high biomass production (high Xm) or because of a very productive strain (high qp) (Aguilar et al. 2001). In this study, it was observed that gallic acid production via a co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT showed more productivity with an average qp of 73.14 µg/mg.h and was also due to the maximum Xm of 4.18 mg/mL at 24 h of fermentation (Table 5). The results of this study are in good pact with the outcomes reported by Aguilar et al. (2001). Certain variations in kinetic parameters and growth rate between the previous reports (Selvaraj et al. 2021; Selvaraj and Vytla, 2017) and the current report may have been caused due to differences in fermentation conditions and microbial species. This is the first report which was discussed the impact of kinetic parameters on gallic acid production via the co-culture method.
Antibacterial activity of gallic acid produced
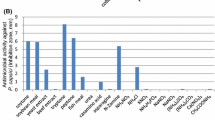
In the food industry, the growth of pathogenic strains of bacteria is the main reason for food spoilage. To combat the adverse effects of food spoilage on health, the search for safe, effective, and natural preservatives has been an integral part of the industry. In this present work, the product gallic acid produced through co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT under submerged fermentation was shown to be natural alternative preservatives for foodstuff and also combats food poisoning, avoiding any health hazards of chemical antimicrobial agent applications. The gallic acid produced under optimized conditions was extracted via cold centrifuge to study the antimicrobial activity. Further investigation of the gallic acid extract was done to estimate the antibacterial activity using the disc diffusion method against four different pathogenic strains of bacteria causing food poisoning—two Gram-positive strains (Bacillus sp. and S. aureus) and two Gram-negative strains (E. coli, and Serriatia marcescens). The strains E. coli, S. aureus, Serriatia marcescens, and Bacillus sp., showed the ZOI of 20.4 ± 0.38, 16.5 ± 0.41, 13.5 ± 0.43, and 09.4 ± 0.32, respectively, against the product gallic acid. The results of the estimation of antibacterial activity are shown in Fig. 3, and indicate that the gallic acid extract is most prominent in subduing the growth of food spoilage bacteria with variable efficacy. Hence, it can be inferred that the gallic acid extract showed robust antibacterial activity and high effectiveness against pathogenic bacteria. Borges et al. (2013) reported the antimicrobial activity of gallic acid against different pathogenic bacteria like Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Listeria monocytogenes.
Conclusion
A maximum gallic acid yield of 574.08 µg/mL was produced after statistical optimization of process conditions, which is a 26- and 27-fold increase when compared to a monoculture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT, respectively, at unoptimized conditions. The results of kinetic studies indicate that production of gallic acid through co-culture of Bacillus gottheilii M2S2 and Bacillus cereus M1GT is completely growth associated and 75% of tannic acid was degraded within 24 h of fermentation. A stable balance between growth and yield coefficients was obtained, which indicates that gallic acid production can be scaled up for commercial use. The fed-batch operation can be implemented to maintain the balance between growth and production. The cell-free extract (gallic acid) has proved to be effective against food spoilage pathogens and can be used as an alternative food preservative to store foodstuffs and control food poisoning diseases.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the first author upon reasonable request.
References
Abdullah R, Naeem N, Aftab M, Kaleem A, Iqtedar M, Iftikhar T, Naz S (2018) Enhanced production of alpha amylase by exploiting novel bacterial co-culture technique employing solid state fermentation. Iran J Sci Technol Trans Sci 42:305–312
Aguilar CN, Augur C, Favela-Torres E, Viniegra-Gonzalez G (2001) Production of tannase by Aspergillus niger Aa-20 in submerged fermentation and solid state fermentation: influence of glucose and tannic acid. J Ind Microbiol Biotechnol 26:296–302
Aguilar-Zarate P, Cruz MA, Montaiez J, Rodriguez-Herrera R, Wong-Paz JE, Belmares RE, Aguilar CN (2015) Gallic acid production under anaerobic submerged fermentation by two bacilli strains. Microb Cell Fact 14:209
Arshad R, Mohyuddin A, Saeed S, Hassan AU (2019) Optimized production of tannase and gallic acid from fruit seeds by solid state fermentation. Trop J Pharm Res 18(5):911–918
Bajpai B, Patil S (2008) A new approach to microbial production of gallic acid. Braz J Microbiol 39(4):708–711
Banerjee R, Mukherjee G, Patra KC (2005) Microbial transformation of tannin-rich substrate to gallic acid through co-culture method. Bioresour Technol 96(8):949–953
Banerjee D, Mahapatra S, Pati BR (2007) Gallic acid production by submerged fermentation of Aspergillus aculeatus DBF9. Res J Microbiol 2:462–468
Belur PD, Mugeraya G (2011) Microbial production of tannase: state of the art. Res J Microbiol 6(1):25–40
Borges A, Ferreira C, Saavedra MJ, Simoes M (2013) Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb Drug Resist 19(4):256–265
Chandrasekaran M, Beena PS (2013) Tannase: source, biocatalytic characteristics, and bioprocesses for production. In: Trincone A (ed) Marine enzymes for biocatalysis. Woodhead publishing, Sawston
Cruz R, de Lima JS, Fonseca JC, Gomes JEG, de Siqueira JI, Moreira KA, de Souza-Motta CM (2017) Promising substrates to increase the production of tannase under solid state fermentation (SSF) by Penicillium spp. Afr J Biotechnol 16:2121–2126
Das Mohapatra PK, Mondal KC, Pati BR (2006) Production of tannase through submerged fermentation of tannin-containing plant extracts by Bacillus licheniformis KBR6. Polish J Microbiol 55(4):297–301
Deschamps AM, Lebeault JM (1984) Production of gallic acid from tara tannin by bacterial strains. Biotechnol Lett 6:237–242
Deschamps A, Otuk G, Lebault J (1983) Production of tannase and degradation of chestnut tannins by bacteria. J Ferment Technol 61:55–59
Doran PM (1995) Homogeneous reactions. Bioprocess engineering principles, 1st edn. Elsevier, California, pp 257–295
Hagerman AE, Butler LG (1978) Protein precipitation method for the quantitative determination of tannins. J Agric Food Chem 26(4):809–812
Kanpiengjai A, Khanongnuch C, Lumyong S, Haltrich D, Nguyen TH, Kittibunchakul S (2020) Co-production of gallic acid and a novel cell-associated tannase by a pigment-producing yeast, Sporidiobolus ruineniae A452. Microb Cell Fact 19:95
Kroes BH, van den Berg AJ, Quarels van Ufford HC, van Dijk H, Labadie RP (1992) Anti-inflammatory activity of gallic acid. Planta Med 58(06):499–504
Li M, Zhao X, Zhang X, Wu D, Leng S (2018) Biodegradation of 17β-estradiol by bacterial co-culture isolated from manure. Sci Rep 8:3787
Lokeshwari N, Sriramireddy D, Pola S, Bobbarala V (2010) Production of an antibacterial drug trimethoprim using novel biotechnological approach. Drug Invent Today 2(5):268–270
Lokeswari N, Sriramireddy D, Pola S, Bobbarala V (2010) Production of gallic acid using mutant strain of Aspergillus oryzae. J Pharm Res 3:1402–1406
Mohan SV, Reddy MV (2013) Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour Technol 128:409–416
Mondal KC, Banerjee D, Banerjee R, Pati BR (2001) Production and characterization of tannase from Bacillus cereus KBR9. J Gen Appl Microbiol 47:263–267
Natarajan K, Rajendran A (2012) Evaluation and optimization of food-grade tannin acyl hydrolase production by a probiotic Lactobacillus plantarum strain in submerged and solid state fermentation. Food Bioprod Process 90(4):780–792
Natarajan K, Rajendran A, Thangavelu V (2011) Effect of culture conditions and kinetic studies on extracellular tannase production by Lactobacillus plantarum MTCC 1407. Indian J Biotechnol 10(3):321–328
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid-state fermentation for the production of industrial enzymes. Curr Sci 77:149–152
Purohit JS, Dutta JR, Nanda RK, Banerjee R (2006) Strain improvement for tannase production from co-culture of Aspergillus foetidus and Rhizopus oryzae. Bioresour Technol 97(6):795–801
Raghuwanshi S, Dutt K, Gupta P, Misra S, Saxena RK (2011) Bacillus sphaericus: the highest bacterial tannase producer with potential for gallic acid synthesis. J Biosci Bioeng 111(6):635–640
Rajamanickam K, Yang J, Sakharkar MK (2019) Gallic acid potentiates the antimicrobial activity of Tulathromycin against two key bovine respiratory disease (BRD) causing-pathogens. Front Pharmacol 9:1486
Saeed S, Aslam S, Mehmood T, Naseer R, Nawaz S, Mujahid H, Firyal S, Anjum AA, Sultan A (2020) Production of gallic acid under solid-state fermentation by utilizing waste from food processing industries. Waste Biomass Valor 12:155–163
Sarjit A, Wang Y, Dykes GA (2015) Antimicrobial activity of gallic acid against thermophilic campylobacter is strain specific and associated with a loss of calcium ions. Food Microbiol 46:227–233
Selvaraj S, Vytla RM (2017) Evaluation of kinetic parameters for growth, tannic acid utilization and tannase production in Bacillus gottheilii M2S2 using polyurethane foam blocks as support. 3-Biotech 7:275
Selvaraj S, Natarajan K, Nowak A, Murty VR (2021) Mathematical modeling and simulation of newly isolated Bacillus cereus M1GT for tannase production through semi-solid state fermentation with agriculture residue Triphala. S Afr J Chem Eng 35:89–97
Selwal MK, Yadav A, Selwal KK, Aggarwal NK, Gupta R, Gautam SK (2010) Optimization of cultural conditions for tannase production by Pseudomonas aeruginosa IIIB 8914 under submerged fermentation. World J Microbiol Biotechnol 26(4):599–605
Sharma S, Bhat TK, Dawra RK (2000) A spectrophotometric method for assay of tannase using rhodanine. Anal Biochem 279(1):85–89
Sharma KP, John PJ, Goswami P, Soni M (2017) Enzymatic synthesis of gallic acid from tannic acid with an inducible hydrolase of Enterobacter spp. Biocatal Biotransform 35(3):177–184
Singh TS, Verma TN (2019) Taguchi design approach for extraction of methyl ester from waste cooking oil using synthesized CaO as heterogeneous catalyst: response surface methodology optimization. Energy Convers Manag 182:383–397
Subbalaxmi S, Murty VR (2016) Process optimization for tannase production by Bacillus gottheilii M2S2 on inert polyurethane foam support. Biocatal Agric Biotechnol 7:48–55
Verma S, Singh A, Mishra A (2013) Gallic acid: molecular rival of cancer. Environ Toxicol Pharmacol 35(3):473–485
Xiao A, Huang Y, Ni H, Cai H, Yang Q (2015) Statistical optimization for tannase production by Aspergillus tubingensis in solid-state fermentation using tea stalks. Electron J Biotechnol 18:143–152
Acknowledgements
The authors show thankfulness to the Department of Biotechnology, Manipal Institute of Technology, Manipal Academy of Higher Education, India; for providing the facilities to carry out the research work.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. No funding was received for the submitted work.
Author information
Authors and Affiliations
Contributions
SS designed the study, performed the optimization, kinetics, and antimicrobial experiments, analyzed the data, and wrote the original draft. JMA contributed to the experimental investigation, data curation, and also in the drafting of the manuscript. VRM supervised the study and was also involved in data interpretation and drafting of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
There is no conflict of interest involved in this research work.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Selvaraj, S., Amaral, J.M. & Murty, V.R. Kinetics and antimicrobial activity of gallic acid by novel bacterial co-culture system using Taguchi’s method and submerged fermentation. Arch Microbiol 204, 584 (2022). https://doi.org/10.1007/s00203-022-03168-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00203-022-03168-2