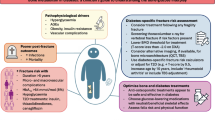
Abstract
This meta-analysis aims to evaluate the impact of Sodium Glucose Transporter 2 (SGLT2) inhibitors on fractures, bone mineral density (BMD), and bone metabolism markers in type 2 diabetes mellitus (T2DM) patients. Pooled relative risk (RR) with 95% confidence interval (CI) assessed the relationship between SGLT2 inhibitors and fracture risk. Weighted mean difference (WMD) with 95% CI explored the correlation between SGLT2 inhibitors and BMD, as well as bone metabolism markers. A total of 20 randomised controlled trials (RCTs) involving 12,764 patients were analysed. No significant association emerged between SGLT2 inhibitor use and elevated fracture risk (pooled RR = 1.21, 95% CI [0.95, 1.54], I2 = 22%). Furthermore, SGLT2 inhibitors exhibited no substantial effects on BMD changes at the lumbar spine (WMD = -0.02, 95% CI [-0.38, 0.34]), femoral neck (WMD = 0.11, 95% CI [-0.28, 0.50]), total hip (WMD = -0.20, 95% CI [-0.41, 0.01]), and distal forearm (WMD = -0.20, 95% CI [-0.62, 0.22]). Similarly, no notable impact of SGLT2 inhibitors on bone metabolism markers, including CTX (WMD = 0.04, 95% CI [-0.02, 0.09]), P1NP (WMD = 1.06, 95% CI [-0.44, 2.57]), PTH (WMD = 0.34, 95% CI [-0.07, 0.75]), calcium (WMD = 0.01, 95% CI [-0.02, 0.04]), and phosphate (WMD = 2.37, 95% CI [-0.76, 5.49]). The findings suggest that the utilization of SGLT2 inhibitors is not significantly linked to an elevated risk of fractures in individuals with T2DM. However, further clinical investigations and extended follow-up periods are warranted to establish more conclusive determinations.








Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author (WWB), upon reasonable request.
References
Sun H, Saeedi P, Karuranga S et al (2022) IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119
Zheng Y, Ley SH, Hu FB (2018) Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 14:88–98
Padhi S, Nayak AK, Behera A (2020) Type II diabetes mellitus: a review on recent drug based therapeutics. Biomed Pharmacother 131:110708
Scheen AJ (2020) Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat Rev Endocrinol 16:556–577
Wu VC, Li YR, Wang CY (2021) Impact of sodium-glucose co-transporter 2 inhibitors on cardiac protection. Int J Mol Sci 22:7170
Lin DS, Lee JK, Chen WJ (2021) Clinical Adverse Events Associated with Sodium-Glucose Cotransporter 2 Inhibitors: A Meta-Analysis Involving 10 Randomized Clinical Trials and 71 553 Individuals. J Clin Endocrinol Metab 106:2133–2145
Ye Y, Zhao C, Liang J, Yang Y, Yu M, Qu X (2018) Effect of Sodium-Glucose Co-transporter 2 Inhibitors on Bone Metabolism and Fracture Risk. Front Pharmacol 9:1517
Adler CP (1989) Pathologic bone fractures: definition and classification. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir 479–486
Watts NB, Bilezikian JP, Usiskin K, Edwards R, Desai M, Law G, Meininger G (2016) Effects of Canagliflozin on Fracture Risk in Patients With Type 2 Diabetes Mellitus. J Clin Endocrinol Metab 101:157–166
Azharuddin M, Adil M, Ghosh P, Sharma M (2018) Sodium-glucose cotransporter 2 inhibitors and fracture risk in patients with type 2 diabetes mellitus: A systematic literature review and Bayesian network meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 146:180–190
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Open Med 3:e123-130
Higgins JPT, Savović J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook. Accessed February 15, 2023.
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–634
Tang HL, Li DD, Zhang JJ, Hsu YH, Wang TS, Zhai SD, Song YQ (2016) Lack of evidence for a harmful effect of sodium-glucose co-transporter 2 (SGLT2) inhibitors on fracture risk among type 2 diabetes patients: a network and cumulative meta-analysis of randomized controlled trials. Diabetes Obes Metab 18:1199–1206
Ljunggren Ö, Bolinder J, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S (2012) Dapagliflozin has no effect on markers of bone formation and resorption or bone mineral density in patients with inadequately controlled type 2 diabetes mellitus on metformin. Diabetes Obes Metab 14:990–999
Bolinder J, Ljunggren Ö, Johansson L, Wilding J, Langkilde AM, Sjöström CD, Sugg J, Parikh S (2014) Dapagliflozin maintains glycaemic control while reducing weight and body fat mass over 2 years in patients with type 2 diabetes mellitus inadequately controlled on metformin. Diabetes Obes Metab 16:159–169
Kohan DE, Fioretto P, Tang W, List JF (2014) Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int 85:962–971
Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, Broedl UC (2014) Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol 2:369–384
Kaku K, Kiyosue A, Inoue S, Ueda N, Tokudome T, Yang J, Langkilde AM (2014) Efficacy and safety of dapagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled by diet and exercise. Diabetes Obes Metab 16:1102–1110
Leiter LA, Cefalu WT, De Bruin TWA, Gause-Nilsson I, Sugg J, Parikh SJ (2014) Dapagliflozin added to usual care in individuals with type 2 diabetes mellitus with preexisting cardiovascular disease: A 24-week, multicenter, randomized, double-blind, placebo-controlled study with a 28-week extension. J Am Geriatr Soc 62:1252–1262
Yale JF, Bakris G, Cariou B et al (2014) Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes mellitus and chronic kidney disease. Diabetes Obes Metab 16:1016–1027
Bode B, Stenlöf K, Harris S, Sullivan D, Fung A, Usiskin K, Meininger G (2015) Long-term efficacy and safety of canagliflozin over 104 weeks in patients aged 55–80 years with type 2 diabetes. Diabetes Obes Metab 17:294–303
Yang W, Han P, Min KW, Wang B, Mansfield T, T’Joen C, Iqbal N, Johnsson E, Ptaszynska A (2016) Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: A randomized controlled trial. J Diabetes 8:796–808
Bilezikian JP, Watts NB, Usiskin K, Polidori D, Fung A, Sullivan D, Rosenthal N (2016) Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J Clin Endocrinol Metab 101:44–51
Araki E, Onishi Y, Asano M, Kim H, Ekholm E, Johnsson E, Yajima T (2016) Efficacy and safety of dapagliflozin in addition to insulin therapy in Japanese patients with type 2 diabetes: Results of the interim analysis of 16-week double-blind treatment period. J Diabetes Inv 7:555–564
Lu CH, Min KW, Chuang LM, Kokubo S, Yoshida S, Cha BS (2016) Efficacy, safety, and tolerability of ipragliflozin in Asian patients with type 2 diabetes mellitus and inadequate glycemic control with metformin: Results of a phase 3 randomized, placebo-controlled, double-blind, multicenter trial. J Diabetes Inv 7:366–373
Rodbard HW, Seufert J, Aggarwal N, Cao A, Fung A, Pfeifer M, Alba M (2016) Efficacy and safety of titrated canagliflozin in patients with type 2 diabetes mellitus inadequately controlled on metformin and sitagliptin. Diabetes Obes Metab 18:812–819
Rosenstock J, Frias J, Páll D et al (2018) Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab 20:520–529
Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, Nakanishi N, Iijima H, Watanabe Y, Gouda M (2017) Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: Results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab 19:874–882
Kaku K, Lee J, Mattheus M, Kaspers S, George J, Woerle HJ (2017) Empagliflozin and cardiovascular outcomes in Asian patients with type 2 diabetes and established cardiovascular disease — results from EMPA-REG OUTCOME® —. Circ J 81:227–234
Yale JF, Xie J, Sherman SE, Garceau C (2017) Canagliflozin in Conjunction With Sulfonylurea Maintains Glycemic Control and Weight Loss Over 52 Weeks: A Randomized, Controlled Trial in Patients With Type 2 Diabetes Mellitus. Clin Ther 39:2230-2242.e2232
Gallo S, Charbonnel B, Goldman A, Shi H, Huyck S, Darekar A, Lauring B, Terra SG (2019) Long-term efficacy and safety of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: 104-week VERTIS MET trial. Diabetes Obes Metab 21:1027–1036
Rau M, Thiele K, Hartmann NK, Möllmann J, Wied S, Hohl M, Marx N, Lehrke M (2022) Effects of empagliflozin on markers of calcium and phosphate homeostasis in patients with type 2 diabetes - Data from a randomized, placebo-controlled study. Bone Rep 16:101175
Song L (2017) Calcium and Bone Metabolism Indices. Adv Clin Chem 82:1–46
Meier C, Schwartz AV, Egger A, Lecka-Czernik B (2016) Effects of diabetes drugs on the skeleton. Bone 82:93–100
Alba M, Xie J, Fung A, Desai M (2016) The effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, on mineral metabolism and bone in patients with type 2 diabetes mellitus. Curr Med Res Opin 32:1375–1385
Blau JE, Taylor SI (2018) Adverse effects of SGLT2 inhibitors on bone health. Nat Rev Nephrol 14:473–474
Taylor SI, Blau JE, Rother KI (2015) Possible adverse effects of SGLT2 inhibitors on bone. Lancet Diabetes Endocrinol 3:8–10
Civitelli R, Armamento-Villareal R, Napoli N (2009) Bone turnover markers: understanding their value in clinical trials and clinical practice. Osteoporos Int 20:843–851
Vasikaran S, Eastell R, Bruyère O et al (2011) Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int 22:391–420
Greenblatt MB, Tsai JN, Wein MN (2017) Bone Turnover Markers in the Diagnosis and Monitoring of Metabolic Bone Disease. Clin Chem 63:464–474
Vilaca T, Gossiel F, Eastell R (2017) Bone Turnover Markers: Use in Fracture Prediction. J Clin Densitom 20:346–352
Szulc P (2018) Bone turnover: Biology and assessment tools. Best Pract Res Clin Endocrinol Metab 32:725–738
Schini M, Vilaca T, Gossiel F, Salam S, Eastell R (2023) Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr Rev 44:417–473
Li X, Li T, Cheng Y, Lu Y, Xue M, Xu L, Liu X, Yu X, Sun B, Chen L (2019) Effects of SGLT2 inhibitors on fractures and bone mineral density in type 2 diabetes: An updated meta-analysis. Diabetes Metab Res Rev 35:e3170
De Laet C, Kanis JA, Odén A et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Lee SW, Han K, Kwon HS (2023) Association of Body Mass Index and Fracture Risk Varied by Affected Bones in Patients with Diabetes: A Nationwide Cohort Study. Diabetes Metab J 47:242–254
Acknowledgements
The research design was carried out by WWB and LYJ. Literature retrieval and screening, data extraction, data analysis, statistical analysis, and written contributions were performed by WX, ZFY, ZYF, and ZJY. The manuscript was revised by WWB and SYL. All authors reviewed the manuscript, and all authors have read and approved the final version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Shandong Province (ZR2020MH361), the Shandong Provincial Traditional Chinese Medicine Science and Technology Project (2020M023), the Shandong Provincial Medical and Health Science and Technology Development Program Project (2019WS562), China Postdoctoral Science Foundation (2021M692750), and Special funding for Mount Tai Scholar Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, F., Zhang, Y. et al. Effect of SGLT2 inhibitors on fractures, BMD, and bone metabolism markers in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Osteoporos Int 34, 2013–2025 (2023). https://doi.org/10.1007/s00198-023-06908-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-023-06908-2