Abstract
Summary
This study showed that autoimmune arthritis induces especially severe osteoporosis in the periarticular region adjacent to inflamed joints, suggesting that arthritis increases the fragility fracture risk near inflamed joints, which is frequently observed in patients with RA.
Introduction
Periarticular osteoporosis near inflamed joints is a hallmark of early rheumatoid arthritis (RA). Here we show that rheumatic inflammation deteriorates the bone quality and bone quantity of periarticular bone, thereby decreasing bone strength and toughness in a mouse model of RA.
Methods
Female BALB/c mice and SKG mice, a mutant mouse model of autoimmune arthritis on the BALB/c background, were used. At 12 weeks of age, BALB/c mice underwent either Sham surgery or bilateral ovariectomy (OVX), and SKG mice underwent intraperitoneal injection of mannan to induce arthritis. Eight weeks later, the mice were killed and the femurs and tibias were subjected to micro-computed tomography, Fourier transform infrared (FTIR) spectroscopic imaging, X-ray diffraction, histology, and mechanical testing.
Results
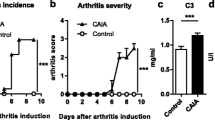
SKG mice developed significant trabecular bone loss in both the distal metaphysis of the femur and the lumbar vertebral body, but the extent of the bone loss was more severe in the distal metaphysis. Neither SKG nor OVX mice exhibited changes in the geometry and matrix properties of the diaphysis of the femur, whereas SKG mice, but not OVX mice, did exhibit changes in these properties in the distal metaphysis of the femur. Bone strength and fracture toughness of the distal metaphysis of the tibia adjacent to the inflamed ankle joint were significantly decreased in SKG mice.
Conclusions
Autoimmune arthritis induces periarticular osteoporosis, characterized by deterioration of cortical bone geometry and quality as well as by trabecular bone loss, leading to severe bone fragility in periarticular bone adjacent to inflamed joints.





Similar content being viewed by others
Abbreviations
- RA:
-
Rheumatoid arthritis
- RANKL:
-
Receptor activator of NF-kB ligand
- SH2:
-
Src homology 2
- ZAP-70:
-
ζ-associated protein of 70 kDa
- CTX:
-
C-terminal telopeptides of type I collagen
- BV/TV:
-
Trabecular bone volume fraction
- Tb.N:
-
Trabecular number
- Tb.Th:
-
Trabecular thickness
- SMI:
-
Structural model index
- Ct.Th:
-
Cortical thickness
- FTIR:
-
Fourier transform infrared
- PMMA:
-
Poly methyl methacrylate
- MCT:
-
Mercury–cadmium–telluride
- MTMR:
-
Mineral to matrix ratio
- CTPR:
-
Carbonate to phosphate ratio
- HAp:
-
Hydroxyapatite
- SD:
-
Standard deviation
References
Alamanos Y, Drosos AA (2005) Epidemiology of adult rheumatoid arthritis. Autoimmun Rev 4(3):130–136. doi:10.1016/j.autrev.2004.09.002
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219. doi:10.1056/NEJMra1004965
van Staa TP, Geusens P, Bijlsma JW, Leufkens HG, Cooper C (2006) Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 54(10):3104–3112. doi:10.1002/art.22117
Orstavik RE, Haugeberg G, Uhlig T, Falch JA, Halse JI, Hoiseth A, Lilleas F, Kvien TK (2003) Vertebral deformities in 229 female patients with rheumatoid arthritis: associations with clinical variables and bone mineral density. Arthritis Rheum 49(3):355–360. doi:10.1002/art.11118
Alenfeld FE, Diessel E, Brezger M, Sieper J, Felsenberg D, Braun J (2000) Detailed analyses of periarticular osteoporosis in rheumatoid arthritis. Osteoporos Int 11(5):400–407. doi:10.1007/s001980070106
Jensen TW, Hansen MS, Horslev-Petersen K, Hyldstrup L, Abrahamsen B, Langdahl B, Zerahn B, Podenphant J, Stengaard-Petersen K, Junker P, Ostergaard M, Lottenburger T, Ellingsen T, Andersen LS, Hansen I, Skjodt H, Pedersen JK, Lauridsen UB, Svendsen AJ, Tarp U, Lindegaard H, Jurik AG, Vestergaard A, Hetland ML (2014) Periarticular and generalised bone loss in patients with early rheumatoid arthritis: influence of alendronate and intra-articular glucocorticoid treatment. Post hoc analyses from the CIMESTRA trial. Ann Rheum Dis 73(6):1123–1129. doi:10.1136/annrheumdis-2012-203171
Rothschild B (2012) Comment on: Periarticular osteoporosis: a useful feature in the diagnosis of early rheumatoid arthritis? Reliability and validity in a cross-sectional diagnostic study using dual-energy X-ray absorptiometry. Rheumatology (Oxford) 51(7):1340–1341. doi:10.1093/rheumatology/kes044, author reply 1341
Iwata T, Ito H, Furu M, Hashimoto M, Fujii T, Ishikawa M, Yamakawa N, Terao C, Azukizawa M, Hamamoto Y, Mimori T, Akiyama H, Matsuda S (2015) Periarticular osteoporosis of the forearm correlated with joint destruction and functional impairment in patients with rheumatoid arthritis. Osteoporos Int. doi:10.1007/s00198-015-3256-1
Jimenez-Boj E, Redlich K, Turk B, Hanslik-Schnabel B, Wanivenhaus A, Chott A, Smolen JS, Schett G (2005) Interaction between synovial inflammatory tissue and bone marrow in rheumatoid arthritis. J Immunol 175(4):2579–2588
Shigeyama Y, Pap T, Kunzler P, Simmen BR, Gay RE, Gay S (2000) Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum 43(11):2523–2530. doi:10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z
Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR (2000) Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum 43(2):250–258. doi:10.1002/1529-0131(200002)43:2<250::AID-ANR3>3.0.CO;2-P
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8(11):656–664. doi:10.1038/nrrheum.2012.153
Brandt KD (2003) Response of joint structures to inactivity and to reloading after immobilization. Arthritis Rheum 49(2):267–271. doi:10.1002/art.11009
Angeli A, Guglielmi G, Dovio A, Capelli G, de Feo D, Giannini S, Giorgino R, Moro L, Giustina A (2006) High prevalence of asymptomatic vertebral fractures in post-menopausal women receiving chronic glucocorticoid therapy: a cross-sectional outpatient study. Bone 39(2):253–259. doi:10.1016/j.bone.2006.02.005
Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S (2003) Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426(6965):454–460. doi:10.1038/nature02119
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486. doi:10.1002/jbmr.141
Boskey A, Pleshko Camacho N (2007) FT-IR imaging of native and tissue-engineered bone and cartilage. Biomaterials 28(15):2465–2478. doi:10.1016/j.biomaterials.2006.11.043
Hasegawa T, Amizuka N, Yamada T, Liu Z, Miyamoto Y, Yamamoto T, Sasaki M, Hongo H, Suzuki R, de Freitas PH, Oda K, Li M (2013) Sclerostin is differently immunolocalized in metaphyseal trabecules and cortical bones of mouse tibiae. Biomed Res 34(3):153–159
Giri B, Tadano S (2011) Nanostructural alteration in bone quantified in terms of orientation distribution of mineral crystals: a possible tool for fracture risk assessment. J Biomech Eng 133(12):124503. doi:10.1115/1.4005482
Caetano-Lopes J, Nery AM, Henriques R, Canhao H, Duarte J, Amaral PM, Vale M, Moura RA, Pereira PA, Weinmann P, Abdulghani S, Souto-Carneiro M, Rego P, Monteiro J, Sakagushi S, Queiroz MV, Konttinen YT, Graca L, Vaz MF, Fonseca JE (2009) Chronic arthritis directly induces quantitative and qualitative bone disturbances leading to compromised biomechanical properties. Clin Exp Rheumatol 27(3):475–482
Keller KK, Thomsen JS, Stengaard-Pedersen K, Dagnaes-Hansen F, Nyengaard JR, Hauge EM (2012) Bone formation and resorption are both increased in experimental autoimmune arthritis. PLoS One 7(12):e53034. doi:10.1371/journal.pone.0053034
Caetano-Lopes J, Nery AM, Canhao H, Duarte J, Cascao R, Rodrigues A, Perpetuo IP, Abdulghani S, Amaral PM, Sakaguchi S, Konttinen YT, Graca L, Vaz MF, Fonseca JE (2010) Chronic arthritis leads to disturbances in the bone collagen network. Arthritis Res Ther 12(1):R9. doi:10.1186/ar2908
Bala Y, Zebaze R, Seeman E (2015) Role of cortical bone in bone fragility. Curr Opin Rheumatol 27(4):406–413. doi:10.1097/BOR.0000000000000183
Gough A, Sambrook P, Devlin J, Huissoon A, Njeh C, Robbins S, Nguyen T, Emery P (1998) Osteoclastic activation is the principal mechanism leading to secondary osteoporosis in rheumatoid arthritis. J Rheumatol 25(7):1282–1289
Skedros JG, Sorenson SM, Takano Y, Turner CH (2006) Dissociation of mineral and collagen orientations may differentially adapt compact bone for regional loading environments: results from acoustic velocity measurements in deer calcanei. Bone 39(1):143–151. doi:10.1016/j.bone.2005.12.007
Reisinger AG, Pahr DH, Zysset PK (2011) Principal stiffness orientation and degree of anisotropy of human osteons based on nanoindentation in three distinct planes. J Mech Behav Biomed Mater 4(8):2113–2127. doi:10.1016/j.jmbbm.2011.07.010
Yamada S, Tadano S, Fujisaki K, Kodaki Y (2013) Influence of osteon area fraction and degree of orientation of HAp crystals on mechanical properties in bovine femur. J Biomech 46(1):31–35. doi:10.1016/j.jbiomech.2012.09.020
Roggia C, Gao Y, Cenci S, Weitzmann MN, Toraldo G, Isaia G, Pacifici R (2001) Up-regulation of TNF-producing T cells in the bone marrow: a key mechanism by which estrogen deficiency induces bone loss in vivo. Proc Natl Acad Sci U S A 98(24):13960–13965. doi:10.1073/pnas.251534698
Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, Garcia I (1997) Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J Clin Invest 99(7):1699–1703. doi:10.1172/JCI119333
Hata H, Sakaguchi N, Yoshitomi H, Iwakura Y, Sekikawa K, Azuma Y, Kanai C, Moriizumi E, Nomura T, Nakamura T, Sakaguchi S (2004) Distinct contribution of IL-6, TNF-alpha, IL-1, and IL-10 to T cell-mediated spontaneous autoimmune arthritis in mice. J Clin Invest 114(4):582–588. doi:10.1172/JCI21795
Acknowledgments
This project was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan 25462357 (M. Takahata) and by a Grant-in-Aid for Scientific Research (A), MEXT (No. 15H02207) (S. Tadano).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The Ethics Review Committee for Animal Experimentation of Hokkaido University approved the experimental protocol of this study.
Conflicts of interest
None.
Rights and permissions
About this article
Cite this article
Shimizu, T., Takahata, M., Kimura-Suda, H. et al. Autoimmune arthritis deteriorates bone quantity and quality of periarticular bone in a mouse model of rheumatoid arthritis. Osteoporos Int 28, 709–718 (2017). https://doi.org/10.1007/s00198-016-3781-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3781-6