Abstract
Summary
Treatment with hydrogen sulfide mitigates spinal cord injury-induced sublesional bone loss, possibly through abating oxidative stress, suppressing MMP activity, and activating Wnt/β-catenin signaling.
Introduction
Spinal cord injury (SCI)-induced sublesional bone loss represents the most severe osteoporosis and is resistant to available treatments to data. The present study was undertaken to explore the therapeutic potential of hydrogen sulfide (H2S) against osteoporosis in a rodent model of motor complete SCI.
Methods
SCI was generated by surgical transaction of the cord at the T3–T4 levels in rats. Treatment with NaHS was initiated through intraperitoneal injection of 0.1 ml/kg/day of 0.28 mol/l NaHS from 12 h following the surgery and over 14 subsequent days.
Results
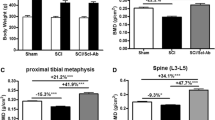
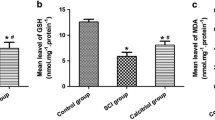
H2S levels in plasma of SCI rats were lower, which was restored by treatment with exogenous H2S. Treatment of SCI rats with exogenous H2S had no significant effect on body mass but increased bone mineral density in femurs and tibiae, increased BV/TV, Tb.Th, and Tb.N and reduced Tb.Sp in proximal tibiae, and increased mineral apposition rate (MAR), bone formation rate (BFR), and osteoblast surface and reduced eroded surface and osteoclast surface in proximal tibiae. More importantly, H2S treatment led to a significant enhancement in ultimate load, stiffness, and energy to max force of femoral diaphysis. Treatment of SCI rats with exogenous H2S reduced malondialdehyde (MDA) levels in serum and femurs, decreased hydroxyproline levels, suppressed activities of matrix metallopeptidase 9 (MMP9), and upregulated Wnt3a, Wnt6, Wnt10, and ctnnb1 expression in femurs.
Conclusion
Treatment with H2S mitigates SCI-induced sublesional bone loss, possibly through abating oxidative stress, suppressing MMP activity, and activating Wnt/β-catenin signaling.






Similar content being viewed by others
References
Gifre L, Vidal J, Carrasco J, Filella X, Ruiz-Gaspà S, Muxi A, Portell E, Monegal A, Guañabens N, Peris P (2015) Effect of recent spinal cord injury on wnt signaling antagonists (sclerostin and Dkk-1) and their relationship with bone loss. A 12-month prospective study. J Bone Miner Res 30:1014–1021
Gifre L, Vidal J, Carrasco J, Portell E, Puig J, Monegal A, Guañabens N, Peris P (2014) Incidence of skeletal fractures after traumatic spinal cord injury: a 10-year follow-up study. Clin Rehabil 28:361–369
Roberts D, Lee W, Cuneo RC, Wittmann J, Ward G, Flatman R, McWhinney B, Hickman PE (1998) Longitudinal study of bone turnover after acute spinal cord injury. J Clin Endocrinol Metab 83:415–422
Warden SJ, KBennell KL, Matthews B, Brown DJ, McMeeken JM, Wark JD (2002) Quantitative ultrasound assessment of acute bone loss following spinal cord injury: a longitudinal pilot study. Osteoporos Int 13:586–592
Vico L, Collet P, Guignandon A, Lafage-Proust MH, Thomas T, Rehaillia M, Alexandre C (2000) Effects of long-term microgravity exposure on cancellous and cortical weight-bearing bones of cosmonauts. Lancet 355:1607–1611
Leblanc AD, Schneider VS, Evans HJ, Engelbretson DA, Krebs JM (1990) Bone mineral loss and recovery after 17 weeks of bed rest. J Bone Miner Res 5:843–850
Recker R, Lappe J, Davies K, Heaney R (2000) Characterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15:1965–1973
Varzi D, Coupaud SA, Purcell M, Allan DB, Gregory JS, Barr RJ (2015) Bone morphology of the femur and tibia captured by statistical shape modelling predicts rapid bone loss in acute spinal cord injury patients. Bone 81:495–501
Minaire P, Berard E, Meunier PJ, Edouard C, Goedert G, Pilonchery G (1981) Effects of disodium dichloromethylene diphosphonate on bone loss in paraplegic patients. J Clin Invest 68:1086–1092
Bryson JE, Gourlay ML (2009) Bisphosphonate use in acute and chronic spinal cord injury: a systematic review. J Spinal Cord Med 32:215–225
Chang KV, Hung CY, Chen WS, Lai MS, Chien KL, Han DS (2013) Effectiveness of bisphosphonate analogues and functional electrical stimulation on attenuating post-injury osteoporosis in spinal cord injury patients-a systematic review and meta-analysis. PLoS One 8:e81124
Bauman WA, Cirnigliaro CM, La Fountaine MF, Martinez L, Kirshblum SC, Spungen AM (2015) Zoledronic acid administration failed to prevent bone loss at the knee in persons with acute spinal cord injury: an observational cohort study. J Bone Miner Metab 33:410–421
Wang R (2003) The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal 5:493–501
Wang R (2002) Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16:1792–1798
Kesherwani V, Nelson KS, Agrawal SK (2013) Effect of sodium hydrosulphide after acute compression injury of spinal cord. Brain Res 1527:222–229
Campolo M, Esposito E, Ahmad A, Di Paola R, Wallace JL, Cuzzocrea S (2013) A hydrogen sulfide-releasing cyclooxygenase inhibitor markedly accelerates recovery from experimental spinal cord injury. FASEB J 27:4489–4499
Grassi F, Malik Tyagi A, Calvert JW, Gambari L, Walker LD, Yu M, Robinson J, Li JY, Lisignoli G, Vaccaro C, Adams J, Pacifici R (2015) Hydrogen Sulfide Is a Novel Regulator of Bone Formation Implicated in the Bone Loss Induced by Estrogen Deficiency. J Bone Miner Res (in press)
Liu Y, Yang R, Liu X, Zhou Y, Qu C, Kikuiri T, Wang S, Zandi E, Du J, Ambudkar IS, Shi S (2014) Hydrogen sulfide maintains mesenchymal stem cell function and bone homeostasis via regulation of Ca(2+) channel sulfhydration. Cell Stem Cell 15:66–78
Qin W, Li X, Peng Y, Harlow LM, Ren Y, Wu Y, Li J, Qin Y, Sun J, Zheng S, Brown T, Feng JQ, Ke HZ, Bauman WA, Cardozo CC (2015) Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res 30:1994–2004
Sun L, Pan J, Peng Y, Wu Y, Li J, Liu X, Qin Y, Bauman WA, Cardozo C, Zaidi M, Qin W (2013) Anabolic steroids reduce spinal cord injury-related bone loss in rats associated with increased wnt signaling. J Spinal Cord Med 36:616–622
Luo ZL, Tang LJ, Wang T, Dai RW, Ren JD, Cheng L, Xiang K, Tian FZ (2014) Effects of treatment with hydrogen sulfide on methionine-choline deficient diet-induced non-alcoholic steatohepatitis in rats. J Gastroenterol Hepatol 29:215–222
Zeng J, Lin X, Fan H, Li C (2013) Hydrogen sulfide attenuates the inflammatory response in a mouse burn injury model. Mol Med Rep 8:1204–1208
Yang X, He B, Liu P, Yan L, Yang M, Li D (2015) Treatment with curcumin alleviates sublesional bone loss following spinal cord injury in rats. Eur J Pharmacol 765:209–216
Wang HD, Shi YM, Li L, Guo JD, Zhang YP, Hou SX (2013) Treatment with resveratrol attenuates sublesional bone loss in spinal cord-injured rats. Br J Pharmacol 170:796–806
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310
Podenphant J, Larsen NE, Christiansen C (1984) An easy and reliable method for determination of urinary hydroxyproline. Clin Chim Acta 142:145–148
Vacek TP, Qipshidze N, Tyagi SC (2013) Hydrogen sulfide and sodium nitroprusside compete to activate/deactivate MMPs in bone tissue homogenates. Vasc Health Risk Manag 9:117–123
Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ (2007) Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J 16:771–776
Lin T, Tong W, Chandra A, Hsu SY, Jia H, Zhu J, Tseng WJ, Levine MA, Zhang Y, Yan SG, Liu XS, Sun D, Young W, Qin L (2015) A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats. Bone Res 3:15028
d’Emmanuele diVilla Bianca R. Mitidieri E, Donnarumma E, Tramontano T, Brancaleone V, Cirino G, Bucci M, Sorrentino R (2015) Hydrogen sulfide is involved in dexamethasone-induced hypertension in rat. Nitric Oxide 46:80–86.
Morimoto E, Li M, Khalid AB, Krum SA, Chimge NO, Frenkel B (2016) Glucocorticoids hijack Runx2 to stimulate Wif1 for suppression of osteoblast growth and differentiation. J Cell Physiol (in press)
Yang M, Huang Y, Chen J, Chen YL, Ma JJ, Shi PH (2014) Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun 454:42–47
Maimoun L, Couret I, Micallef JP, Peruchon E, Mariano-Goulart D, Rossi M, Leroux JL, Ohanna F (2002) Use of bone biochemical markers with dual-energy x-ray absorptiometry for early determination of bone loss in persons with spinal cord injury. Metabolism 51:958–963
Itou T, Maldonado N, Yamada I, Goettsch C, Matsumoto J, Aikawa M, Singh S, Aikawa E (2014) Cystathionine γ-lyase accelerates osteoclast differentiation: identification of a novel regulator of osteoclastogenesis by proteomic analysis. Arterioscler Thromb Vasc Biol 34:626–634
Gambari L, Lisignoli G, Cattini L, Manferdini C, Facchini A, Grassi F (2014) Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via NRF2-dependent mechanism. Pharmacol Res 87:99–112
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359–370
Jia Z, Zhu H, Li J, Wang X, Misra H, Li Y (2012) Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 50:264–274
Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, Bian JS (2011) Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med 50:1314–1323
Williams S, Barnes J, Wakisaka A, Ogasa H, Liang CT (1999) Treatment of osteoporosis with MMP inhibitors. Ann N Y Acad Sci 878:191–200
Siwik DA, Pagano PJ, Colucci WS (2001) Oxidative stress regulates collagen synthesis and matrix metalloproteinase activity in cardiac fibroblasts. Am J Physiol Cell Physiol 280:C53–C60
Rodda SJ, McMahon AP (2006) Distinct roles for hedgehog and canonical wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development 133:3231–3244
Glass DA 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Beggs LA, Ye F, Ghosh P, Beck DT, Conover CF, Balaez A, Miller JR, Phillips EG, Zheng N, Williams AA, Aguirre JI, Wronski TJ, Bose PK, Borst SE, Yarrow JF (2015) Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res 30:681–689
Fan K, Li N, Qi J, Yin P, Zhao C, Wang L, Li Z, Zha X (2014) Wnt/β-catenin signaling induces the transcription of cystathionine-γ-lyase, a stimulator of tumor in colon cancer. Cell Signal 26:2801–2808
Si YF, Wang J, Guan J, Zhou L, Sheng Y, Zhao J (2013) Treatment with hydrogen sulfide alleviates streptozotocin-induced diabetic retinopathy in rats. Br J Pharmacol 169:619–631
Li N, Wang MJ, Jin S, Bai YD, Hou CL, Ma FF, Li XH, Zhu YC (2016) The H2S donor NaHS changes the expression pattern of H2S-producing enzymes after myocardial infarction. Oxidative Med Cell Longev 2016:6492469
Ahmad A, Sattar MA, Rathore HA, Abdulla MH, Khan SA, Azam M, Abdullah NA, Johns EJ (2016) Up regulation of cystathione γ lyase and hydrogen Sulphide in the myocardium inhibits the progression of isoproterenol-caffeine induced left ventricular hypertrophy in Wistar Kyoto rats. PLoS One 11:e0150137
Jiang SD, Dai LY, Jiang LS (2006) Osteoporosis after spinal cord injury. Osteoporos Int 17:180–192
Yang M, Huang Y, Chen J, Chen YL, Ma JJ, Shi PH (2007) Activation of AMPK participates hydrogen sulfide-induced cyto-protective effect against dexamethasone in osteoblastic MC3T3-E1 cells. Biochem Biophys Res Commun 454:42–47
Jain SK, Manna P, Micinski D, Lieblong BJ, Kahlon G, Morehead L, Hoeldtke R, Bass PF 3rd, Levine SN (2013) In African American type 2 diabetic patients, is vitamin D deficiency associated with lower blood levels of hydrogen sulfide and cyclic adenosine monophosphate, and elevated oxidative stress? Antioxid Redox Signal 18:1154–1158
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Electronic supplementary material
ESM 1
(DOC 49 kb)
Rights and permissions
About this article
Cite this article
Yang, X., Hao, D., Zhang, H. et al. Treatment with hydrogen sulfide attenuates sublesional skeletal deterioration following motor complete spinal cord injury in rats. Osteoporos Int 28, 687–695 (2017). https://doi.org/10.1007/s00198-016-3756-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-016-3756-7