Abstract
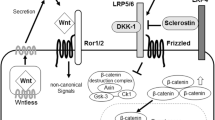
Osteocytes, entrapped within a newly mineralized bone matrix, possess a unique cellular identity due to a specialized morphology and a molecular signature. These features endow them to serve as a bone response mechanism for mechanical stress in their microenvironment. Sclerostin, a primarily osteocyte product, is widely considered as a mechanotranduction key molecule whose expression is suppressed by mechanical loading, or it is induced by unloading. This review presents a model suggesting that sclerostin is major mediator for integrating mechanical, local, and hormonal signals, sensed by the osteocytes, in controlling the remodeling apparatus. This central role is achieved through interplay between two opposing mechanisms: (1) unloading-induced high sclerostin levels, which antagonize Wnt-canonical-β-catenin signaling in osteocytes and osteoblasts, permitting simultaneously Wnt-noncanonical and/or other pathways in osteocytes and osteoclasts, directed at bone resorption; (2) mechanical loading results in low sclerostin levels, activation of Wnt-canonical signaling, and bone formation. Therefore, adaptive bone remodeling occurring at a distinct bone compartment is orchestrated by altered sclerostin levels, which regulate the expression of the other osteocyte-specific proteins, such as RANKL, OPG, and proteins encoded by “mineralization-related genes” (DMP1, PHEX, and probably FGF23). For example, under specific terms, sclerostin regulates differential RANKL and OPG production, and creates a dynamic RANKL/OPG ratio, leading either to bone formation or resorption. It also controls the expression of PHEX, DMP1, and most likely FGF23, leading to either bone matrix mineralization or its inhibition. Such opposing up- or down-regulation of remodeling phases allows osteocytes to function as an “external unit”, ensuring transition from bone resorption to bone formation.
Mini Abstract: The osteocyte network plays a central role in directing bone response either to mechanical loading, or to unloading, leading correspondingly to bone formation or resorption. This review shows a key role of the osteocyte-produced sclerostin as a major mediator of the molecular mechanisms involved in the process of adaptive bone remodeling



Similar content being viewed by others
References
Sims NA, Martin TJ (2014) Coupling the activities of bone formation and resorption: a multitude of signals within the basic multicellular unit. BoneKey Rep 3:481. doi:10.1038/bonekey.2013.215
Cranney A, Jamal SA, Tsang JF, Josse RG, Leslie WD (2007) Low bone mineral density and fracture burden in postmenopausal women. CMAJ 177:575–580
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285:25103–25108
Feng X, McDonald JM (2011) Disorders of bone remodeling. Ann Rev Pathol Mech Dis 6:121–145
Kular J, Tickner J, Chim SM, Xu J (2012) An overview of the regulation of bone remodelling at the cellular level. Clin Biochem 45:863–873
Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G, Rosen V, Erber W, Xu J (2013) Angiogenic factors in bone local environment. Cytokine Growth Factor Rev 24:297–310
Parfitt AM (2008) Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. In: Marcus R, Feldman D, Nelson DA, Rosen CJ (eds) Osteoporosis. Elsevier, San Diego, pp 71–92
Franz-Odendaal TA, Hall BK, Witten PE (2006) Buried alive: how osteoblasts become osteocytes. Dev Dyn 235:176–190
Bonewald LF (2007) Osteocytes as dynamic multifunctional cells. Ann N Y Acad Sci 1116:281–290
Knothe Tate ML, Niederer P, Knothe U (1998) In vivo tracer transport through the lacunocanalicular system of rat bone in an environment devoid of mechanical loading. Bone 22:107–117
Kamioka H, Sugawara Y, Honjo T, Yamashiro T, Takano-Yamamoto T (2004) Terminal differentiation of osteoblasts to osteocytes is accompanied by dramatic changes in the distribution of actin-binding proteins. J Bone Miner Res 19:471–478
Bellido T (2014) Osteocyte-driven bone remodeling. Calcif Tissue Int 94:25–34
Skerry TM (2008) The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch Biochem Biophys 473:117–123
Rubin CT, Lanyon LE (1987) Kappa delta award paper. Osteoregulatory nature of mechanical stimuli: function as a determinant for adaptive remodeling in bone. J Orthop Res 5:300–310
Cowin SC, Sadegh AM, Luo GM (1992) An evolutionary Wolff’s law for trabecular architecture. J Biomech Eng 114:129–136
Wang X, Dumas GA (2002) Simulation of bone adaptive remodeling using a stochastic process as loading history. J Biomech 35:375–380
Skerry TM (2006) One mechanostat or many? Modifications of the site-specific response of bone to mechanical loading by nature and nurture. J Musculoskelet Neuronal Interact 6:122–127
McNamaraa LM, Van der Lindenb JC, Weinansb H, Prendergast PJ (2006) Stress-concentrating effect of resorption lacunae in trabecular bone. J Biomech 39:734–741
Smit TH, Burger EH (2000) Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res 15:301–307
Prendergast PJ, Huiskes R (1996) Microdamage and osteocyte-lacuna strain in bone: a microstructural finite element analysis. J Biomech Eng 118:240–246
Mori S, Burr DB (1993) Increased intracortical remodeling following fatigue damage. Bone 14:103–109
Van der Linden JC, Homminga J, Verhaar JA, Weinans H (2001) Mechanical consequences of bone loss in cancellous bone. J Bone Miner Res 16:457–465
Dallas SL, Prideaux M, Bonewald LF (2013) The osteocyte: an endocrine cell … and more. Endocr Rev. doi:10.1210/er.2012-1026
Rochefort GY, Pallu S, Benhamou CL (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21:1457–1469
Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S (2013) Mechanosensation and transduction in osteocytes. Bone 54:182–190
Delgado-Calle J, Sanudo C, Bolado A, Fernandez A, Arozamena J, Pascual-Carra MA, Rodriguez-Rey JC, Fraga M, Bonewald LF, Riancho JA (2012) DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J Bone Miner Res 27:926–937
Balemans W, Ebeling M, Patel N, Van Hul E, Olson P, Dioszegi M, Lacza C, Wuyts W, Van Den Ende J, Willems P, Paes-Alves AF, Hill S, Bueno M, Ramos FJ, Tacconi P, Dikkers FG, Stratakis C, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Hum Mol Genet 10:537–543
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39:91–97
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23:860–869
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/betacatenin signaling. J Bone Miner Res 24:1651–1661
Collette NM, Genetos DC, Economides AN, Xie L, Shahnazari M, Yao W, Lane NE, Harland RM, Loots GG (2012) Targeted deletion of Sost distal enhancer increases bone formation and bone mass. Proc Natl Acad Sci U S A 109:14092–14097
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22:6267–6276
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50:209–217
Lewiecki EM (2009) Emerging drugs for postmenopausal osteoporosis. Expert Opin Emerg Drugs 14:129–144
Padhi D, Jang G, Stouch B, Fang L, Posvar E (2011) Single-dose, placebo-controlled, randomized study of AMG 785, a sclerostin monoclonal antibody. J Bone Miner Res 26:19–26
McColm J, Hu L, Womack T, Tang CC, Chiang AY (2014) Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res 29:935–943
Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, Dwyer D, Stolina M, Ke HZ, Bouxsein ML (2013) Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res 28:865–874
Papapoulos SE (2011) Targeting sclerostin as potential treatment of osteoporosis. Ann Rheum Dis 70(Suppl 1):i119–i122
Li X, Grisanti M, Fan W, Asuncion FJ, Tan HL, Dwyer D, Han CY, Yu L, Lee J, Lee E, Barrero M, Kurimoto P, Niu QT, Geng Z, Winters A, Horan T, Steavenson S, Jacobsen F, Chen Q, Haldankar R, Lavallee J, Tipton B, Daris M, Sheng J, Lu HS, Daris K, Deshpande R, Valente EG, Salimi-Moosavi H, Kostenuik PJ, Li J, Liu M, Li C, Lacey DL, Simonet WS, Ke HZ, Babij P, Stolina M, Ominsky MS, Richards WG (2011) Dickkopf-1 regulates bone formation in young growing rodents and upon traumatic injury. J Bone Miner Res 26:2610–2621
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Sapir-Koren R, Livshits G (2011) Bone mineralization and regulation of phosphate homeostasis. IBMS BoneKEy 8:286–300
Bonewald LF (2011) The amazing osteocyte. J Bone Miner Res 26:229–238
Guo R, Rowe PS, Liu S, Simpson LG, Xiao ZS, Quarles LD (2002) Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Commun 297:38–45
Liu S, Rowe PS, Vierthaler L, Zhou J, Quarles LD (2007) Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol 192:261–267
Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD (2006) Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291:E38–E49
Feng JQ, Clinkenbeard EL, Yuan B, White KE, Drezner MK (2013) Osteocyte regulation of phosphate homeostasis and bone mineralization underlies the pathophysiology of the heritable disorders of rickets and osteomalacia. Bone 54:213–221
Hamdy NA (2007) Targeting the RANK/RANKL/OPG signaling pathway: a novel approach in the management of osteoporosis. Curr Opin Investig Drugs 8:299–303
Xu J, Wu HF, Ang ES, Yip K, Woloszyn M, Zheng MH, Tan RX (2009) Nf-kappab modulators in osteolytic bone diseases. Cytokine Growth Factor Rev 20:7–17
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Kramer I, Halleux C, Keller H, Pegurri M, Gooi JH, Weber PB, Feng JQ, Bonewald LF, Kneissel M (2010) Osteocyte Wnt/β-catenin signaling is required for normal bone homeostasis. Mol Cell Biol 30:3071–3085
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Zhao S, Kato Y, Zhang YK, Harris S, Ahuja SS, Bonewald LF (2002) MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res 17:2068–2079
Kamioka H, Honjo T, Takano-Yamamoto T (2001) A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone 28:145–149
Honma M, Ikebuchi Y, Kariya Y, Hayashi M, Hayashi N, Aoki S, Suzuki H (2013) RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J Bone Miner Res 28:1936–1949
Corral DA, Amling M, Priemel M, Loyer E, Fuchs S, Ducy P, Baron R, Karsenty G (1998) Dissociation between bone resorption and bone formation in osteopenic transgenic mice. Proc Natl Acad Sci U S A 95:13835–13840
Galli C, Fu Q, Wang W, Olsen BR, Manolagas SC, Jilka RL, O’Brien CA (2009) Commitment to the osteoblast lineage is not required for RANKL gene expression. J Biol Chem 284:12654–12662
Xiong J, O’Brien CA (2012) Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res 27:499–505
Pierroz DD, Bonnet N, Baldock PA, Ominsky MS, Stolina M, Kostenuik PJ, Ferrari SL (2010) Are osteoclasts needed for the bone anabolic response to parathyroid hormone? A study of intermittent parathyroid hormone with denosumab or alendronate in knock-in mice expressing humanized RANKL. J Biol Chem 285:28164–28173
Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, Burnett-Bowie S-AM, Neer RM, Leder BZ (2013) Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet 382:50–56
Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20:781–810
Gordon MD, Nusse R (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem 281:22429–22433
Maeda K, Takahashi N, Kobayashi Y (2013) Roles of Wnt signals in bone resorption during physiological and pathological states. J Mol Med 91:15–23
Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L (2002) Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in LRP5, a Wnt coreceptor. J Cell Biol 157:303–314
Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR, Zhong Z, Matthes S, Jacobsen CM, Conlon RA, Brommage R, Liu Q, Mseeh F, Powell DR, Yang QM, Zambrowicz B, Gerrits H, Gossen JA, He X, Bader M, Williams BO, Warman ML, Robling AG (2011) LRP5 functions in bone to regulate bone mass. Nat Med 17:684–691
Zhao L, Shim JW, Dodge TR, Robling AG, Yokota H (2013) Inactivation of Lrp5 in osteocytes reduces young’s modulus and responsiveness to the mechanical loading. Bone 54:35–43
Glass DA II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G (2005) Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev Cell 8:751–764
Holmen SL, Zylstra CR, Mukherjee A, Sigler RE, Faugere MC, Bouxsein ML, Deng L, Clemens TL, Williams BO (2005) Essential role of beta-catenin in postnatal bone acquisition. J Biol Chem 280:21162–21168
Pederson L, Ruan M, Westendorf JJ, Khosla S, Oursler MJ (2008) Regulation of bone formation by osteoclasts involves Wnt/BMP signaling and the chemokine sphingosine-1-phosphate. Proc Natl Acad Sci U S A 105:20764–20769
Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y (2011) Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol 31:4706–4719
Bonewald LF, Johnson ML (2008) Osteocytes, mechanosensing and Wnt signaling. Bone 42:606–615
Maeda K, Kobayashi Y, Udagawa N, Uehara S, Ishihara A, Mizoguchi T, Kikuchi Y, Takada I, Kato S, Kani S, Nishita M, Marumo K, Martin TJ, Minami Y, Takahashi N (2012) Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nat Med 18:405–412
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
van Bezooijen RL, Roelen BA, Visser A, van der Wee-Pals L, de Wilt E, Karperien M, Hamersma H, Papapoulos SE, ten Dijke P, Löwik CW (2004) Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J Exp Med 199:805–814
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J 19:1842–1844
Balemans W, Piters E, Cleiren E, Ai M, Van Wesenbeeck L, Warman ML, Van Hul W (2008) The binding between sclerostin and LRP5 is altered by DKK1 and by high-bone mass LRP5 mutations. Calcif Tissue Int 82:445–453
Heino TJ, Kurata K, Higaki H, Vaananen HK (2009) Evidence for the role of osteocytes in the initiation of targeted remodeling. Technol Health Care 17:49–56
van Oers RF, van Rietbergen B, Ito K, Hilbers PA, Huiskes R (2011) A sclerostin-based theory for strain-induced bone formation. Biomech Model Mechanobiol 10:663–670
Tian X, Jee WS, Li X, Paszty C, Ke HZ (2011) Sclerostin antibody increases bone mass by stimulating bone formation and inhibiting bone resorption in a hindlimb-immobilization rat model. Bone 48:197–201
Moustafa A, Sugiyama T, Prasad J, Zaman G, Gross TS, Lanyon LE, Price JS (2012) Mechanical loading-related changes in osteocyte sclerostin expression in mice are more closely associated with the subsequent osteogenic response than the peak strains engendered. Osteoporos Int 23:1225–1234
Ryan ZC, Ketha H, McNulty MS, McGee-Lawrence M, Craig TA, Grande JP, Westendorf JJ, Singh RJ, Kumar R (2013) Sclerostin alters serum vitamin D metabolite and fibroblast growth factor 23 concentrations and the urinary excretion of calcium. PNAS 110:6199–6204
Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253
Semenov M, Tamai K, He X (2005) SOST is a ligand for LRP5/LRP6 and a WNT signaling inhibitor. J Biol Chem 280:26770–26775
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–197887
Ellies DL, Viviano B, McCarthy J, Rey JP, Itasaki N, Saunders S, Krumlauf R (2006) Bone density ligand, sclerostin, directly interacts with LRP5 but not LRP5G171V to modulate Wnt activity. J Bone Miner Res 21:1738–1749
Krause C, Korchynskyi O, de Rooij K, Weidauer SE, de Gorter DJJ, van Bezooijen RL, Hatsell S, Economides AN, Mueller TD, Löwik CW, ten Dijke P (2010) Distinct modes of inhibition by sclerostin on bone morphogenetic protein and Wnt signaling pathways. J Biol Chem 285:41614–41626
Sutherland MK, Geoghegan JC, Yu C, Winkler DG, Latham JA (2004) Unique regulation of SOST, the sclerosteosis gene, by BMPs and steroid hormones in human osteoblasts. Bone 35:448–454
Moester MJC, Papapoulos SE, Lowik CW, van Bezooijen RL (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87:99–107
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH (2006) The Wnt co-receptor Lrp5 is essential for skeletal mechanotransduction, but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281:23698–23711
Galea GL, Sunters A, Meakin LB, Zaman G, Sugiyama T, Lanyon LE, Price JS (2011) Sost down-regulation by mechanical strain in human osteoblastic cells involves PGE2 signaling via EP4. FEBS Lett 585:2450–2454
Sapir-Koren R, Livshits G (2013) Is interaction between age-dependent decline in mechanical stimulation and osteocyte–estrogen receptor levels the culprit for postmenopausal-impaired bone formation? Osteoporos Int 24:1771–1789
Yadav VK, Ryu JH, Suda N, Tanaka KF, Gingrich JA, Schutz G, Glorieux FH, Chiang CY, Zajac JD, Insogna KL, Mann JJ, Hen R, Ducy P, Karsenty G (2008) Lrp5 controls bone formation by inhibiting serotonin synthesis in the duodenum. Cell 135:825–837
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24:578–588
Tian X, Setterberg RB, Li X, Paszty C, Ke HZ, Jee WS (2010) Treatment with a sclerostin antibody increases cancellous bone formation and bone mass regardless of marrow composition in adult female rats. Bone 47:529–533
Wijenayaka AR, Kogawa M, Lim HP, Bonewald LF, Findlay DM, Atkins GJ (2011) Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS One 6:e25900
You L, Temiyasathit S, Lee P, Kim CH, Tummala P, Yao W, Kingery W, Malone AM, Kwon RY, Jacobs CR (2008) Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 42:172–179
Kogawa M, Wijenayaka AR, Ormsby R, Thomas GP, Anderson PH, Bonewald LF, Findlay DM, Atkins JF (2013) Sclerostin regulates release of bone mineral by osteocytes by induction of carbonic anhydrase 2. J Bone Miner Res 28:2436–2448
Ito N, Findlay DM, Anderson PH, Bonewald LF, Atkins GJ (2013) Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J Steroid Biochem Mol Biol 136:183–186
Zhou X, Cui Y, Zhou X, Han J (2012) Phosphate/pyrophosphate and MV-related proteins in mineralization: discoveries from mouse models. Int J Biol Sci 8:778–790
Camalier CE, Yi M, Yu LR, Hood BL, Conrads KA, Lee YJ, Lin Y, Garneys LM, Bouloux GF, Young MR, Veenstra TD, Stephens RM, Colburn NH, Conrads TP, Beck GR Jr (2013) An integrated understanding of the physiological response to elevated extracellular phosphate. J Cell Physiol 228:1536–1550
Rendenbach C, Yorgan TA, Heckt T, Otto B, Baldauf C, Jeschke A, Streichert T, David JP, Amling M, Schinke T (2014) Effects of extracellular phosphate on gene expression in murine osteoblasts. Calcif Tissue Int 94:473–483
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE, Pavlin D (2003) Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 18:807–817
Yang W, Lu Y, Kalajzic I, Guo D, Harris MA, Gluhak-Heinrich J, Kotha S, Bonewald LF, Feng JQ, Rowe DW, Turner CH, Robling AG, Harris SE (2005) Dentin matrix protein 1 gene cis regulation: use in osteocytes to characterize local responses to mechanical loading in vitro and in vivo. J Biol Chem 280:20680–20690
Harris SE, Gluhak-Heinrich J, Harris MA, Yang W, Bonewald LF, Riha D, Rowe PSN, Robling AG, Turner CH, Feng JQ, McKee MD, Nicollela D (2007) DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact 7:313–315
Addison WN, Nakano Y, Loisel T, Crine P, McKee MD (2008) MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res 23:1638–1649
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2010) Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int 87:461–468
Atkins JG, Rowe PS, Lim HP, Welldon KJ, Ormsby R, Wijenayaka AR, Zelenchuk L, Evdokiou A, Findlay DM (2011) Sclerostin is a locally acting regulator of late-osteoblast/preosteocyte differentiation and regulates mineralization through a MEPE-ASARM-dependent mechanism. J Bone Miner Res 26:1425–1436
Mackenzie NCW, Zhu D, Milne EM, van’t Hof R, Martin A, Quarles DL, Millán JL, Farquharson C, MacRae VE (2012) Altered bone development and an increase in FGF-23 expression in Enpp1 (−/−) mice. PLoS ONE 7:e32177
Ärnlöv J, Carlsson AC, Sundström J, Ingelsson E, Larsson A, Lind L, Larsson TE (2013) Higher fibroblast growth factor-23 increases the risk of all-cause and cardiovascular mortality in the community. Kidney Int 83:160–166
Fang Y, Ginsberg C, Sugatani T, Monier-Faugere MC, Malluche H, Hruska KA (2014) Early chronic kidney disease–mineral bone disorder stimulates vascular calcification. Kidney Int 85:142–150
Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Müller R, Kesavan C, Sheng MH (2013) Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab 305:E271–E281
Cohen-Kfir E, Artsi H, Levin A, Abramowitz E, Bajayo A, Gurt I, Zhong L, D’Urso A, Toiber D, Mostoslavsky R, Dresner-Pollak R (2011) Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology 152:4514–4524
Ohyama Y, Nifuji A, Maeda Y, Amagasa T, Noda M (2004) Spaciotemporal association and bone morphogenetic protein regulation of sclerostin and osterix expression during embryonic osteogenesis. Endocrinology 145:4685–4692
Milat F, Ng KW (2009) Is Wnt signalling the final common pathway leading to bone formation? Mol Cell Endocrinol 310:52–62
van Bezooijen RL, Papapoulos S, Hamdy AT, Lowik CW (2008) SOST/sclerosin: an osteocyte-derived inhibitor of bone formation that antagonizes canonical Wnt signaling. In: Bilezikian JP, Raisz LG, Martin TJ (eds) Principles of bone biology. Academic, San Diego, pp 139–152
Lloyd SA, Loiselle AE, Zhang Y, Donahue HJ (2013) Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 57:76–83
Kramer I, Loots GG, Studer A, Keller H, Kneissel M (2010) Parathyroid hormone (PTH)-induced bone gain is blunted in SOST overexpressing and deficient mice. J Bone Miner Res 25:178–189
Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin RJ, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM (2010) Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metab 11:161–171
Powell WF Jr, Barry KJ, Tulum I, Kobayashi T, Harris SE, Bringhurst FR, Pajevic PD (2011) Targeted ablation of the PTH/PTHrP receptor in osteocytes impairs bone structure and homeostatic calcemic responses. J Endocrinol 209:21–32
Mirza FS, Padhi ID, Raisz LG, Lorenzo JA (2010) Serum sclerostin levels negatively correlate with parathyroid hormone levels and free estrogen index in postmenopausal women. J Clin Endocrinol Metab 95:1991–1997
Modder UI, Clowes JA, Hoey K, Peterson JM, McCready L, Oursler MJ, Riggs BL, Khosla S (2011) Regulation of circulating sclerostin levels by sex steroids in women and in men. J Bone Miner Res 26:27–34
Kim B-J, Bae SJ, Lee S-Y Lee Y-S, Baek J-E, Park S-Y, Lee SH, Koh J-M, Kim GS (2012) TNF-a mediates the stimulation of sclerostin expression in an estrogen-deficient condition. Biochem Biophys Res Commun 424:170–175
Leupin O, Kramer I, Collette NM, Loots GG, Natt F, Kneissel M, Keller H (2007) Control of the SOST bone enhancer by PTH using MEF2 transcription factors. J Bone Miner Res 22:1957–1967
Roberts MD, Santner TJ, Hart RT (2009) Local bone formation due to combined mechanical loading and intermittent hPTH-(1-34) treatment and its correlation to mechanical signal distributions. J Biomech 42:2431–2438
Devarajan-Ketha H, Craig TA, Madden BJ, Robert Bergen H 3rd, Kumar R (2012) The sclerostin-bone protein interactome. Biochem Biophys Res Commun 417:830–835
Lewiecki EM (2014) Role of sclerostin in bone and cartilage and its potential as a therapeutic target in bone diseases. Ther Adv Musculoskelet Dis 6:48–57
Ominsky MS, Niu QT, Li C, Li X, Ke HZ (2014) Tissue-level mechanisms responsible for the increase in bone formation and bone volume by sclerostin antibody. J Bone Miner Res 29:1424–1430
Ross RD, Edwards LH, Acerbo AS, Ominsky MS, Virdi AS, Sena K, Miller LM, Sumner DR (2014) Bone matrix quality following sclerostin antibody treatment. J Bone Miner Res 29:1597–1607
Acknowledgments
This study was funded by Israel Science Foundation, grant #994/10 and grant #1018/13. The study was also supported by Dr. Herman Showeder fund from the Sackler Faculty of Medicine, Tel Aviv University.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sapir-Koren, R., Livshits, G. Osteocyte control of bone remodeling: is sclerostin a key molecular coordinator of the balanced bone resorption–formation cycles?. Osteoporos Int 25, 2685–2700 (2014). https://doi.org/10.1007/s00198-014-2808-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-014-2808-0