Abstract
Summary
To characterize the changes in osteoprotegerin-deficient (OPG−/−) mice mandibles and the possible mandibular bone loss prevention by zoledronate. This preventive effect in the mandible differed from that in the proximal tibia and was independent of the OPG pathway.
Introduction
The study aimed to characterize both the changes in the mandible in osteoprotegerin-deficient (OPG−/−) mice and possible mandibular bone loss prevention by zoledronate.
Methods
Twenty-eight 6-week-old female mice (C57BL/6J), including OPG−/− (n = 21) and wild-type (WT) (n = 7) mice, were assigned to four groups after 2 weeks of acclimatization to local vivarium conditions: wild mice with vehicle (WT group); OPG−/− mice with vehicle (OPG−/− group); and OPG−/− mice that were subcutaneously injected with either 50 or 150 μg/kg zoledronate (Zol-50 and Zol-150 groups, respectively). Mice were sacrificed at 4 weeks after these treatments and after fasting for 12 h. Sera were harvested for biochemical analyses. The right mandible and tibia of each mouse were selected for microCT analysis. Student’s t-test was performed for comparisons of bone parameters at different sites in the WT group. Analysis of variance (ANOVA) was used to compare the biomarkers and bone parameters in the different treatment groups.
Results
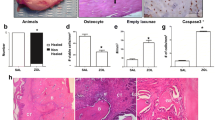
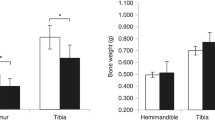
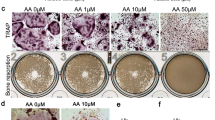
Serum bone-specific alkaline phosphatase (B-ALP) and tartrate-resistant acid phosphatase 5b (TRACP-5b) were significantly decreased in WT mice as compared to the levels in the OPG−/− mice (P < 0.05). Zoledronate treatment decreased the high serum B-ALP activity observed in OPG−/− mice to the levels seen in WT mice, while serum TRACP-5b concentrations were decreased to levels even lower than those in WT mice. There were substantial variations in BMD and microstructure of the mandibular and proximal tibial trabeculae. Mandibular bone loss was less affected by OPG gene deprivation than the proximal tibia was. Both zoledronate groups showed greater BMD, trabecular BV/TV, Tb.Th, Tb.N, and Conn.D and a significant decrease in Tb.Sp and SMI as compared to the findings in OPG−/− mice (P < 0.05). However, higher apparent BMD and more compact plate-like trabeculae were observed in the mandible after treatment with zoledronate as compared to the findings in the proximal tibia. No significant differences were found in any parameter in both zoledronate groups.
Conclusions
The present study showed that zoledronate could reverse the significant bone loss in mice mandibles that was induced by OPG gene deficiency. This preventive effect, which was accompanied with considerable inhibition of bone turnover, differed in the mandible and in the proximal tibia and was independent of the OPG pathway.





Similar content being viewed by others
References
Felsenberg D, Boonen S (2005) The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 27:1–11
Turner CH (2002) Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int 13:97–104
Jeffcoat M (2005) The association between osteoporosis and oral bone loss. J Periodontol 76(Suppl):2125–2132
Krall EA, Garcia RI, Dawson-Hughes B (1996) Increased risk of tooth loss is related to bone loss at the whole body, hip, and spine. Calcif Tissue Int 59:433–437
Taguchi A, Tanimoto K, Suei Y, Wada T (1995) Tooth loss and mandibular osteopenia. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 79:127–132
Kribbs PJ (1990) Comparison of mandibular bone in normal and osteoporotic women. J Prosthet Dent 63:218–222
Payne JB, Reinhardt RA, Nummikoski PV, Patil KD (1999) Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int 10:34–40
Taguchi A, Tanimoto K, Suei Y, Otani K, Wada T (1995) Oral signs as indicators of possible osteoporosis in elderly women. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 80:612–616
Taguchi A, Sanada M, Krall E, Nakamoto T, Ohtsuka M, Suei Y, Tanimoto K, Kodama I, Tsuda M, Ohama K (2003) Relationship between dental panoramic radiographic findings and biochemical markers of bone turnover. J Bone Miner Res 18:1689–1694
Simonet WS, Lacey DL, Danstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ (1997) Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 89:309–319
Kong YY, Yoshida H, Sarosi L, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM (1999) OPGL is a key regulation of osteoclastogenesis, lymphocyte development and lymphnode organogensis. Nature 397:315–323
Teitelbaum SL (2000) Bone resorption by osteoclasts. Science 289:1504–1508
Hofbauer LC, Khosla S, Dunstan CR, Lacey DL, Boyle WJ, Riggs BL (2000) The role of osteoprotegerin and osteoprotegrin ligand in the paracrine regulation of bone resorption. J Bone Miner Res 15:2–12
Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, Scully S, Tan HL, Xu W, Lacey DL, Boyle WJ, Simonet WS (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 29(247):610–615
Nakamura M, Udagawa N, Matsuura S, Mogi M, Nakamura H, Horiuchi H, Saito N, Hiraoka BY, Kobayashi Y, Takaoka K, Ozawa H, Miyazawa H, Takahashi N (2003) Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology 144:5441–5449
Jiang G, Matsumoto H, Yamane J, Kuboyama N, Akimoto Y, Fujii A (2004) Prevention of trabecular bone loss in the mandible of ovariectomized rats. J Oral Sci 46:75–85
Kimura E, Nishioka T, Hasegawa K, Maki K (2007) Effects of bisphosphonate on the mandible of rats in the growing phase with steroid-induced osteoporosis. Oral Dis 13:544–549
Srisubut S, Teerakapong A, Vattraphodes T, Taweechaisupapong S (2007) Effect of local delivery of alendronate on bone formation in bioactive glass grafting in rats. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 10:e11–e16
Li EC, Davis LE (2003) Zoledronic acid: a new parenteral bisphosphonate. Clin Ther 25:2669–2708
Reinholz GG, Getz B, Pederson L, Sanders ES, Subramaniam M, Ingle JN, Spelsberg TC (2000) Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res 60:6001–6007
Green JR (2001) Chemical and biological prerequisites for novel bisphosphonate molecules: Results of comparative preclinical studies. Semin Oncol 28(Suppl):4–10
Mavropoulos A, Rizzoli R, Ammann P (2007) Different responsiveness of alveolar and tibial bone to bone loss stimuli. J Bone Miner Res 22:403–410
Yamashiro T, Takano-Yamamoto T (1998) Differential responses of mandibular condyle and femur to oestrogen deficiency in young rats. Arch Oral Biol 43:191–195
Mizuno A, Amizuka N, Irie K, Murakami A, Fujise N, Kanno T, Sato Y, Nakagawa N, Yasuda H, Mochizuki S, Gomibuchi T, Yano K, Shima N, Washida N, Tsuda E, Morinaga T, Higashio K, Ozawa H (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Tommasini SM, Morgan TG, van der Meulen MCh, Jepsen KJ (2005) Genetic variation in structure-function relationships for the inbred mouse lumbar vertebral body. J Bone Miner Res 20:817–827
Sheng ZF, Dai RC, Wu XP, Fang LN, Fan HJ, Liao EY (2007) Regionally specific compensation for bone loss in the tibial trabeculae of estrogen-deficient rats. Acta Radiol 48:531–539
Jiang G, Matsumoto H, Fujii A (2003) Mandible bone loss in osteoporosis rats. J Bone Miner Metab 21:388–395
Mavropoulos A, Kiliaridis S, Bresin A, Ammann P (2004) Effect of different masticatory functional and mechanical demands on the structural adaptation of the mandibular alveolar bone in young growing rats. Bone 35:191–197
Green JR (2004) Bisphosphonates: preclinical review. Oncologist 9(Suppl):3–13
Yong Hee K, Gwan-shik K, Jeong-Hwa B (2002) Inhibitory action of bisphosphonates on bone resorption does not involve the regulation of RANKL and OPG expression. Exp Mol Med 34:145–151
Mackie PS, Fisher JL, Zhou H, Choong PF (2001) Bisphosphonates regulate cell growth and gene expression in the UMR 106–01 clonal rat osteosarcoma cell line. Br J Cancer 84:951–958
Viereck V, Emons G, Lauck V, Frosch KH, Blaschke S, Gründker C, Hofbauer LC (2002) Bisphosphonates pamidronate and zoledronic acid stimulate osteoprotegerin production by primary human osteoblasts. Biochem Biophys Res Commun 291:680–686
Pan B, Farrugia AN, To LB, Findlay DM, Green J, Lynch K, Zannettino AC (2004) The nitrogen-containing bisphosphonate, zoledronic acid, influences RANKL expression in human osteoblast-like cells by activating TNF-alpha converting enzyme (TACE). J Bone Miner Res 19:147–154
Rhee Y, Won YY, Baek MH, Lim SK (2004) Maintenance of increased bone mass after recombinant human parathyroid hormone (1–84) with sequential zoledronate treatment in ovariectomized rats. J Bone Miner Res 19:931–937
Glatt M, Pataki A, Evans GP, Hornby SB, Green JR (2004) Loss of vertebral bone and mechanical strength in estrogen-deficient rats is prevented by long-term administration of zoledronic acid. Osteoporos Int 15:707–715
Marx RE, Cillo JE Jr, Ulloa JJ (2007) Oral Bisphosphonate-Induced Osteonecrosis: Risk Factors, Prediction of Risk Using Serum CTX Testing, Prevention, and Treatment. J Oral Maxillofac Surg 65:2397–2410
Dannemann C, Grätz KW, Riener MO, Zwahlen RA (2007) Jaw osteonecrosis related to bisphosphonate therapy: a severe secondary disorder. Bone 40:828–834
Durie BG, Katz M, Crowley J (2005) Osteonecrosis of the jaw and bisphosphonates. N Engl J Med 353:99–102 discussion 99–102
Bamias A, Kastritis E, Bamia C, Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D, Anagnostopoulos A, Papadimitriou C, Terpos E, Dimopoulos MA (2005) Osteonecrosis of the jaw in cancer after treatment with bisphosphonates: incidence and risk factors. J Clin Oncol 23:8580–8587
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 30400514 and No.30470430), and the Ministry of Health P.R. of China (No. 2004–468–50). The authors thank Ms Suan Joanna, Mr Zengxing Liu and Dr. Zou Ke (GE Healthcare Company, Canada) for their assistance with technology supporting.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Drs. Sheng and Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Sheng, ZF., Xu, K., Ma, YL. et al. Zoledronate reverses mandibular bone loss in osteoprotegerin-deficient mice. Osteoporos Int 20, 151–159 (2009). https://doi.org/10.1007/s00198-008-0640-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-008-0640-0