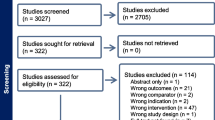
Abstract
Introduction and hypothesis
We hypothesized that patients with refractory overactive bladder (rOAB) have similar improvement with percutaneous tibial nerve stimulation (PTNS) and OnabotulinumtoxinA (BTX).
Methods
This multicenter cohort study compared BTX and PTNS in women with rOAB. Baseline information included Overactive Bladder Questionnaire (OABq) short form, Urinary Distress Inventory-6 (UDI-6), and voiding diary. Primary outcome was cure, defined as “very much better” or “much better” on the Patient Global Impression of Improvement (PGII) AND a reduction in OABq symptom severity scale (SSS) ≥10 at 3 months after treatment. Assuming 80% power to detect a ten-point difference in OABq-SSS, 80 participants were required per group.
Results
A total of 150 patients were enrolled; 97 completed 3 months of therapy and were included. At baseline, BTX patients had more detrusor overactivity (70% vs 40%, p = 0.025), urgency incontinence (UUI; OABq-SSS#6 4 vs 3, p = 0.02, SSS 65 vs 56, p = 0.04), but similar health-related quality of life (HRQL 49 vs 54, p = 0.28), voids (7 vs 8, p = 0.13), and UUI episodes (2 vs 2, p = 1.0). At 3 months, cure rates were similar: BTX 50% vs PTNS 44.2% (p = 0.56). Both groups had improved SSS (−37 vs −29, p = 0.08) and HRQL (31 vs 24, p = 0.14). Patients receiving BTX had a greater improvement in urgency (ΔOABq-SSS#2–3 vs −2; p = 0.02) and UUI (ΔOABq-SSS#6–2 vs -1; p = 0.02). No characteristics were predictive of cure.
Conclusions
BTX resulted in significantly greater improvement in urgency and UUI than PTNS, but no difference in success based on PGII and OABq-SSS, which may be due to a lack of power.

Similar content being viewed by others
References
Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4–20. https://doi.org/10.1002/nau.20798.
Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline. J Urol. 2014;188(6 Suppl):2455–63
Wang M, Jian Z, Ma Y, et al. Percutaneous tibial nerve stimulation for overactive bladder syndrome: a systematic review and meta-analysis. Int Urogynecol J. 2020;31(12):2457-71. https://doi.org/10.1007/s00192-020-04429-8.
Peters KM, Carrico DJ, Wooldridge LS, et al. Percutaneous tibial nerve stimulation for the long-term treatment of overactive bladder: 3-year results of the STEP study. J Urol. 2013;189:2194–201. https://doi.org/10.1016/j.juro.2012.11.175.
Duthie JB, Vincent M, Herbison GP, et al. Botulinum toxin injections for adults with overactive bladder syndrome. Cochrane Database Syst Rev. 2011;(12):CD005493.
Cruz F, Nitti V. Chapter 5: clinical data in neurogenic detrusor overactivity (NDO) and overactive bladder (OAB). Neurourol Urodyn. 2014;33(Suppl 3):S26–31. https://doi.org/10.1002/nau.22630.
Allergan Pharmaceuticals Highlights of prescribing information. 2013. Available from: http://www.allergan.com/assets/pdf/botox_pi.pdf.
Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189:98–101. https://doi.org/10.1067/mob.2003.379.
Tincello D, Owen R, Slack M, Abrams K. Validation of the patient global impression scales for use in detrusor overactivity: secondary analysis of the RELAX study. BJOG. 2013;120:212–6. https://doi.org/10.1111/1471-0528.12069.
Coyne KS, Matza LS, Thompson CL, et al. Determining the importance of change in the overactive bladder questionnaire. J Urol. 2006;176:627–32. https://doi.org/10.1016/j.juro.2006.03.088.
Amundsen CL, Richter HE, Menefee SA, et al. OnabotulinumtoxinA vs sacral neuromodulation on refractory urgency urinary incontinence in women. JAMA. 2016;316:1366–74. https://doi.org/10.1001/jama.2016.14617.
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs. OnabotulinumtoxinA for urgency urinary incontinence. N Engl J Med. 2012;367:1803–13. https://doi.org/10.1056/NEJMoa1208872.
Peters KM, Carrico DJ, Perez-Marrero RA, et al. Randomized trial of percutaneous tibial nerve stimulation versus sham efficacy in the treatment of overactive bladder syndrome: results from the SUmiT trial. J Urol. 2010;183:1438–43. https://doi.org/10.1016/j.juro.2009.12.036.
Finazzi-Agrò E, Petta F, Sciobica F, et al. Percutaneous tibial nerve stimulation effects on detrusor overactivity incontinence are not due to a placebo effect: a randomized, double-blind, placebo controlled trial. J Urol. 2010;184:2001–6. https://doi.org/10.1016/j.juro.2010.06.113.
Balk EM, Rofeberg VN, Adam GP, et al. Pharmacologic and nonpharmacologic treatments for urinary incontinence in women: a systematic review and network meta-analysis of clinical outcomes. Ann Intern Med. 2019;170:465. https://doi.org/10.7326/M18-3227.
Funding
IUGA Uroplasty 2015 Research Grant: Drs. Cheryl Iglesia and Katelyn Smithling Kopcsay.
MedStar Washington Hospital Center Graduate Medical Education Research grant (#2014–277).
Author information
Authors and Affiliations
Contributions
K.S. Kopcsay: project development, data collection, manuscript writing; A.P. Cameron: project development, data collection, manuscript editing; R. Khavari: project development, data collection, manuscript editing; R.E. Gutman: project development, data collection, manuscript editing; T.D. Marczak: data collection, manuscript editing; P.C. Jeppson: data collection, manuscript editing; E. Tefera: data analysis.
Corresponding author
Ethics declarations
Financial disclaimer/conflicts of interest
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
AUGS/IUGA Joint Scientific Meeting 2019, Nashville, TN 9/25/19, USA
Patient preference question
Patient preference question

Rights and permissions
About this article
Cite this article
Kopcsay, K.S., Marczak, T.D., Jeppson, P.C. et al. Treatment of refractory overactive bladder with OnabotulinumtoxinA vs PTNS: TROOP trial. Int Urogynecol J 33, 851–860 (2022). https://doi.org/10.1007/s00192-021-05030-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-021-05030-3