Abstract
Purpose
To assess the efficacy and safety of betrixaban for venous thromboembolism (VTE) prophylaxis among critically ill patients.
Methods
The APEX trial randomized 7513 acutely ill hospitalized patients to betrixaban for 35–42 days or enoxaparin for 10 ± 4 days. Among those, 703 critically ill patients admitted to the intensive care unit were included in the analysis, and 547 patients who had no severe renal insufficiency or P-glycoprotein inhibitor use were included in the full-dose stratum. The risk of VTE, bleeding, net clinical benefit (composite of VTE and major bleeding), and mortality was compared at 35–42 days and at 77 days.
Results
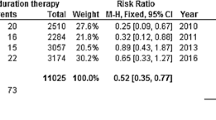
At 35–42 days, extended betrixaban reduced the risk of VTE (4.27% vs 7.95%, P = 0.042) without causing excess major bleeding (1.14% vs 3.13%, P = 0.07). Both VTE (3.32% vs 8.33%, P = 0.013) and major bleeding (0.00% vs 3.26%, P = 0.003) were decreased in the full-dose stratum. Patients who received betrixaban had more non-major bleeding than enoxaparin (overall population: 2.56% vs 0.28%, P = 0.011; full-dose stratum: 3.32% vs 0.36%, P = 0.010). Mortality was similar at the end of study (overall population: 13.39% vs 16.19%, P = 0.30; full-dose stratum: 13.65% vs 16.30%, P = 0.39).
Conclusions
Compared with shorter-duration enoxaparin, critically ill medical patients who received extended-duration betrixaban had fewer VTE without more major bleeding events. The benefit of betrixaban was driven by preventing asymptomatic thrombosis and offset by an elevated risk of non-major bleeding. The APEX trial did not stratify by intensive care unit admission and the present study included a highly selected population of critically ill patients. These hypothesis-generating findings need to be validated in future studies.
Clinical trial registration
http://www.clinicaltrials.gov. Unique identifier: NCT01583218.


Similar content being viewed by others
Abbreviations
- CrCl:
-
Creatinine clearance
- DVT:
-
Deep vein thrombosis
- ICU:
-
Intensive care unit
- PE:
-
Pulmonary embolism
- ULN:
-
Upper limit of normal
- VTE:
-
Venous thromboembolism
References
Minet C, Potton L, Bonadona A, Hamidfar-Roy R, Somohano CA, Lugosi M, Cartier JC, Ferretti G, Schwebel C, Timsit JF (2015) Venous thromboembolism in the ICU: main characteristics, diagnosis and thromboprophylaxis. Crit Care 19:287
Geerts W, Cook D, Selby R, Etchells E (2002) Venous thromboembolism and its prevention in critical care. J Crit Care 17:95–104
Cade JF (1982) High risk of the critically ill for venous thromboembolism. Crit Care Med 10:448–450
Kapoor M, Kupfer YY, Tessler S (1999) Subcutaneous heparin prophylaxis significantly reduces the incidence of venous thromboembolic events in the critically ill. Crit Care Med 27:A69
Fraisse F, Holzapfel L, Couland JM, Simonneau G, Bedock B, Feissel M, Herbecq P, Pordes R, Poussel JF, Roux L, The Association of Non-University Affiliated Intensive Care Specialist Physicians of France (2000) Nadroparin in the prevention of deep vein thrombosis in acute decompensated COPD. Am J Respir Crit Care Med 161:1109–1114
Spyropoulos AC, Anderson FA Jr, Fitzgerald G, Decousus H, Pini M, Chong BH, Zotz RB, Bergmann J-F, Tapson V, Froehlich JB (2011) Predictive and associative models to identify hospitalized medical patients at risk for VTE. Chest 140:706–714
Decousus H, Tapson VF, Bergmann J-F, Chong BH, Froehlich JB, Kakkar AK, Merli GJ, Monreal M, Nakamura M, Pavanello R (2011) Factors at admission associated with bleeding risk in medical patients: findings from the improve investigators. Chest 139:69–79
Goldhaber S, Kett D, Cusumano C, Kuroki C (2000) Low molecular weight heparin versus minidose unfractionated heparin for prophylaxis against venous thromboembolism in medical intensive care unit patients: a randomized controlled trial. J Am Coll Cardiol 35(2 suppl A):325A2000
PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group, Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O, Vlahakis NE (2011) Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 364:1305–1314
Amin AN, Varker H, Princic N, Lin J, Thompson S, Johnston S (2012) Duration of venous thromboembolism risk across a continuum in medically ill hospitalized patients. J Hosp Med 7:231–238
Heit JA, Crusan DJ, Ashrani AA, Petterson TM, Bailey KR (2017) Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood 130:109–114
Cohen AT, Harrington RA, Goldhaber SZ, Hull RD, Wiens BL, Gold A, Hernandez AF, Gibson CM, APEX Investigators (2016) Extended thromboprophylaxis with betrixaban in acutely ill medical patients. N Engl J Med 375:534–544
Chi G, Gibson CM, Hernandez AF, Hull RD, Cohen AT, Harrington RA, Alkhalfan F, Kalayci A, Kerneis M, Nafee T, Goldhaber SZ, APEX Investigators (2018) Betrixaban versus enoxaparin for venous thromboembolism prophylaxis in critically ill patients: findings from the APEX trial. Eur Heart J 39:Ehy563.4321–Ehy4563.4321
Schulman S, Kearon C, Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis (2005) Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost 3:692–694
Kaatz S, Ahmad D, Spyropoulos AC, Schulman S, Subcommittee on Control of Anticoagulation (2015) Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost 13:2119–2126
Sr Kahn, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, LE H, Schulman S, Murad MH (2012) Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:E195s–E226s
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, de Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, Mcintyre LA, Mclean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van Der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP (2017) Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 43:304–377
Cohen AT, Harrington R, Goldhaber SZ, Hull R, Gibson CM, Hernandez AF, Kitt MM, Lorenz TJ (2014) The design and rationale for the acute medically ill venous thromboembolism prevention with extended duration betrixaban (APEX) study. Am Heart J 167:335–341
Raskob GE, Spyropoulos AC, Zrubek J, Ageno W, Albers G, Elliott CG, Halperin J, Haskell L, Hiatt WR, Maynard GA, Peters G, Spiro T, Steg PG, Suh EY, Weitz JI (2016) The mariner trial of rivaroxaban after hospital discharge for medical patients at high risk of VTE. Design, rationale, and clinical implications. Thromb Haemost 115:1240–1248
Gibson CM, Spyropoulos AC, Cohen AT, Hull RD, Goldhaber SZ, Yusen Rd, Hernandez AF, Korjian S, Daaboul Y, Gold A, Harrington RA, Chi G (2017) The IMPROVEDD VTE risk score: incorporation of d-dimer into the IMPROVE score to improve venous thromboembolism risk stratification. Th Open 1:E56–E65
Spyropoulos AC, Ageno W, Albers GW, Elliott CG, Halperin JI, Hiatt WR, Maynard GA, Steg PG, Weitz JI, Suh E, Spiro TE, Barnathan ES, Raskob GE, MARINER Investigators (2018) Rivaroxaban for thromboprophylaxis after hospitalization for medical illness. N Engl J Med 379:1118–1127
Lippi G, Tripodi A, Simundic AM, Favaloro EJ (2015) International survey on d-dimer test reporting: a call for standardization. Semin Thromb Hemost 41:287–293
Weitz JI, Fredenburgh JC, Eikelboom JW (2017) A test in context: d-dimer. J Am Coll Cardiol 70:2411–2420
Guyatt GH, Eikelboom JW, Gould MK, Garcia DA, Crowther M, Murad MH, Kahn SR, Falck-Ytter Y, Francis CW, Lansberg MG, Akl EA, Hirsh J (2012) Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:E185s–E194s
European Medicines Agency (2016) Guideline on clinical investigation of medicinal products for prevention of venous thromboembolism (VTE) in non-surgical patients (Formerly CPMP/EWP/6235/04). EMA, London
Kearon C (2003) Natural history of venous thromboembolism. Circulation 107:I22–I30
Markel A (2005) Origin and natural history of deep vein thrombosis of the legs. Semin Vasc Med 5(1):65–74.
Eikelboom JW, Quinlan DJ, Douketis JD (2001) Extended-duration prophylaxis against venous thromboembolism after total hip or knee replacement: a meta-analysis of the randomised trials. Lancet 358:9–15
Hull RD, Pineo GF, Stein PD, Mah AF, Macisaac SM, Dahl OE, Butcher M, Brant RF, Ghali WA, Bergqvist D, Raskob GE (2001) Extended out-of-hospital low-molecular-weight heparin prophylaxis against deep venous thrombosis in patients after elective hip arthroplasty: a systematic review. Ann Intern Med 135:858–869
Cohen AT, Bailey CS, Alikhan R, Cooper DJ (2001) Extended thromboprophylaxis with low molecular weight heparin reduces symptomatic venous thromboembolism following lower limb arthroplasty–a meta-analysis. Thromb Haemost 85:940–941
Vaitkus PT, Leizorovicz A, Cohen AT, Turpie AG, Olsson CG, Goldhaber SZ, PREVENT Medical Thromboprophylaxis Study Group (2005) Mortality rates and risk factors for asymptomatic deep vein thrombosis in medical patients. Thromb Haemost 93:76–79
Kalayci A, Gibson CM, Chi G, Mk Yee, Korjian S, Datta S, Nafee T, Gurin M, Haroian N, Qamar I, Hull RD, Hernandez AF, Cohen AT, Harrington RA, Goldhaber SZ (2018) Asymptomatic deep vein thrombosis is associated with an increased risk of death: insights from the APEX trial. Thromb Haemost 118:2046–2052
Chi G, Goldhaber SZ, Kittelson JM, Turpie AGG, Hernandez AF, Hull RD, Gold A, Curnutte JT, Cohen AT, Harrington RA, Gibson CM (2017) Effect of extended-duration thromboprophylaxis on venous thromboembolism and major bleeding among acutely ill hospitalized medical patients: a bivariate analysis. J Thromb Haemost 15:1913–1922
Bergqvist D, Agnelli G, Cohen AT, Eldor A, Nilsson PE, Le Moigne-Amrani A, Dietrich-Neto F, ENOXACAN II Investigators (2002) Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med 346:975–980
Eriksson BI, Lassen MR, PENTasaccharide in HIp-FRActure Surgery Plus Investigators (2003) Duration of prophylaxis against venous thromboembolism with fondaparinux after hip fracture surgery: a multicenter, randomized, placebo-controlled, double-blind study. Arch Intern Med 163:1337–1342
Chi G, Goldhaber SZ, Hull RD, Hernandez AF, Kerneis M, Al Khalfan F, Cohen AT, Harrington RA, Gibson CM (2017) Thrombus burden of deep vein thrombosis and its association with thromboprophylaxis and d-dimer measurement: insights from the APEX trial. Thromb Haemost 117:2389–2395
Chan NC, Bhagirath V, Eikelboom JW (2015) Profile of betrixaban and its potential in the prevention and treatment of venous thromboembolism. Vasc Health Risk Manag 11:343–351
Levy JH, Douketis J, Weitz JI (2018) Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol 15:273–281
Hutchaleelaha A, Ye C, Song Y, Lorenz T, Gretler D, Lambing JL (2012) Metabolism and disposition of betrixaban and its lack of interaction with major CYP enzymes. Blood 120:2266
Cook DJ, Griffith LE, Walter SD, Guyatt GH, Meade MO, Heyland DK, Kirby A, Tryba M, Canadian Critical Care Trials Group (2001) The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 5:368–375
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM (2014) Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383:955–962
Bagshaw SM, Stelfox HT, Iwashyna TJ, Bellomo R, Zuege D, Wang X (2018) Timing of onset of persistent critical illness: a multi-centre retrospective cohort study. Intensive Care Med 44:2134–2144
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The study was sponsored by Portola Pharmaceuticals, Inc. The funding source had no role in (1) design and conduct of the study; (2) collection, management, analysis, and interpretation of the data; (3) preparation, review, or approval of the manuscript; and (4) decision to submit and disseminate the results for publication. Dr. Chi receives modest research grant support paid to the Beth Israel Deaconess Medical Center, Harvard Medical School from Portola Pharmaceuticals, Bayer, and Janssen Scientific Affairs. Dr. Gibson receives consultant fees from Portola Pharmaceuticals and reports grants from Angel Medical Corporation and CSL Behring; grants and other support from Bayer Corporation; grants and personal fees from Janssen, Johnson & Johnson, and Portola Pharmaceuticals; and personal fees from The Medicines Company, Boston Clinical Research Institute, Cardiovascular Research Foundation, Eli Lilly, Gilead Sciences Inc, Novo Nordisk, Pfizer, Web MD, UpToDate in Cardiovascular Medicine, Amarin Pharma, Amgen, Arena Pharmaceuticals, Bayer Corporation, Boehringer Ingelheim, Chiesi, Merck & Co, PharmaMar, Sanofi, Somahlution, St Francis Hospital, and Verreseon Corporation. Dr. Cohen reports grant support, personal fees, and non-financial support from Portola Pharmaceuticals during the conduct of the study; grant support, personal fees, and non-financial support from Daiichi–Sankyo, Bristol–Myers Squibb, Pfizer, Janssen, and Bayer Pharmaceuticals, personal fees from Boehringer Ingelheim and Sanofi, and personal fees and non-financial support from Johnson & Johnson and Aspen Pharmaceuticals outside the submitted work. Dr. Hernandez reports receipt of grant support from Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Luitpold, Merck, and Novartis; and personal fees from Amgen, AstraZeneca, Bayer, Bristol–Myers Squibb, Boston Scientific, Luitpold, and Novartis outside the submitted work. Dr. Hull reports grant support from Portola Pharmaceuticals during the conduct of the study, and grant support and personal fees from Leo Pharma outside the submitted work. Dr. Harrington reports grant support from Portola Pharma during the conduct of the study; grant support from CSL Behring, AstraZeneca, GlaxoSmithKline, Regado, and Sanofi Aventis, grant support and personal fees from Merck and The Medicines Company, personal fees from Amgen, Gilead Sciences, MyoKardia, and WebMD, and other support from Scanadu, SignalPath, Element Science, Vida Health, and Adverse Events outside the submitted work. Dr. Goldhaber has provided consulting for Boehringer Ingelheim, Bayer, Portola, Daiichi–Sankyo, Janssen, BiO2 Medical, EKOS/BTG, BMS, and Zafgen. All remaining authors declare no conflicts of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chi, G., Gibson, C.M., Kalayci, A. et al. Extended-duration betrixaban versus shorter-duration enoxaparin for venous thromboembolism prophylaxis in critically ill medical patients: an APEX trial substudy. Intensive Care Med 45, 477–487 (2019). https://doi.org/10.1007/s00134-019-05565-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-019-05565-6