Abstract
Purpose
Recombinant human erythropoietin (rhEPO) attenuated ischemia/reperfusion (I/R) injury-induced spinal cord damage. Since carbamylated EPO derivatives are stated to be devoid of rhEPO side effects, we tested the hypothesis that a newly developed carbamylated EPO-FC fusion protein (cEPO-FC) would compare favorably with rhEPO.
Methods
Anesthetized and mechanically ventilated pigs randomly received cEPO-FC (50 μg kg−1), rhEPO (5,000 IU kg−1) or vehicle (n = 9 per group) 30 min prior to 30 min of aortic occlusion and over the 4 h of reperfusion. During aortic occlusion, mean arterial pressure (MAP) was maintained at 80–120% of baseline values by esmolol, nitroglycerin, and adenosine-5′-triphosphate (ATP). During reperfusion, noradrenaline was titrated to keep MAP at pre-ischemic levels. Spinal cord function was assessed by motor evoked potentials (MEP) and lower limb reflexes. Tissue damage was evaluated using hematoxylin and eosin, Nissl, and terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) staining. Plasma levels of interleukin-6, tumor necrosis factor-α, and 8-isoprostanes were measured as markers of systemic inflammation and oxidative stress.
Results
While only cEPO-FC restored MEP amplitude to values close to pre-occlusion levels, both cEPO-FC and rhEPO comparably restored lower limb reflexes and reduced the percentage of damaged neurons. Infiltration of mononuclear inflammatory cells was moderate without intergroup difference; positive TUNEL staining was barely detectable in any group. I/R injury increased blood cytokine levels without intergroup difference, whereas both cEPO-FC and rhEPO significantly lowered 8-isoprostane levels.
Conclusions
In a porcine model of aortic balloon occlusion-induced spinal cord I/R injury, cEPO-FC and rhEPO comparably protected against ischemic spinal cord dysfunction and neuronal damage. This effect coincided with attenuated oxidative stress.
Similar content being viewed by others
Introduction
Thoracic aortic cross-clamping during aortic aneurysm repair is a typical clinical example of ischemia/reperfusion (I/R) injury. The most vulnerable organs are the kidneys and the spinal cord [1]. Recombinant human erythropoietin (rhEPO) protected against spinal cord I/R injury [2, 3], and, in fact, we previously showed in swine that 300 IU kg−1 rhEPO prior to aortic balloon occlusion and during early reperfusion reduced both thoracic spinal cord neuronal damage and glial apoptosis. Motor neuron function, however, did not recover, most likely due to the low dose of rhEPO administered and/or as a result of the marked tissue damage [4]. Moreover, Ehrenreich et al. [5] recently reported a significantly higher overall death rate in rhEPO-treated patients with stroke, possibly as a result of the high number of patients undergoing systemic thrombolysis with recombinant tissue plasminogen activator: in vitro, a single rhEPO exposure enhances production of plasminogen activator inhibitor-1 [6].
The hematopoietic effects of rhEPO have been shown to be through the activation of a homodimeric EPO receptor complex (EPO-R/EPO-R), whereas organ-protective properties are referred to activation of an alternative receptor complex consisting of the EPO-R and the common β receptor (EPO-R/βcR) [7]. Stimulation of EPO-R/βcR alone was reported to be devoid of the undesired EPO side effects attributed to activation of the EPO-R/EPO-R complex [8, 9]. Carbamylated EPO derivatives (cEPO) do not bind to EPO-R/EPO-R, but are as cytoprotective as rhEPO [9, 10]. Thus, they represent an interesting alternative to rhEPO [10]: cEPO showed neuroprotective activity in models of traumatic brain injury [11] and stroke [12]. Moreover, cEPO was recently reported to more effectively reduce kidney inflammation in brain-dead rats than rhEPO [13]. All data on cEPO-related organ protection against I/R injury, however, originate from nonresuscitated rodent models which did not integrate standard resuscitation measures such as fluid resuscitation and/or vasopressor support to guarantee well-maintained systemic hemodynamics. Therefore, we tested the hypothesis that a newly developed carbamylated EPO-FC fusion protein, consisting of two EPO molecules fused to the FC part of IgG1 (cEPO-FC) [14], protects against ischemic spinal cord dysfunction and neuronal damage comparably to rhEPO in swine undergoing thoracic aortic balloon occlusion-induced I/R injury [4].
Materials and methods
Animals
The experiments were performed in adherence to National Institutes of Health Guidelines on the Use of Laboratory Animals. The experimental protocol had been approved by the University Animal Care Committee and the Federal Authorities for animal research.
Surgical preparation
Anesthesia and surgical instrumentation were performed as in previous experiments [4, 15]. After induction of anesthesia with iv propofol and ketamine and subsequent endotracheal intubation, anesthesia was maintained with continuous iv propofol (6–8 mg kg−1 h−1) and remifentanil (15–20 µg kg−1 h−1). It was carefully checked that the propofol and remifentanil infusion rates were identical in all experimental groups. Pigs were mechanically ventilated [FiO2 0.35, tidal volume 8 mL kg−1, positive end-expiratory pressure (PEEP) 10 cmH2O, inspiratory/expiratory time ratio 1:1.5, respiratory rate 13–15 min−1 adjusted to maintain arterial PCO2 = 35–45 mmHg]. These ventilator settings were used because swine are particularly susceptible to atelectasis formation in dependent lung regions due to the lack of alveolar collateral ventilation [16]. Normothermia (37.5–38.5°C) was maintained with heating pads. Sodium heparin (1,000 IU h−1) was continuously infused for anticoagulation. Via surgical cut-downs, catheters were placed in the A. carotis dextra for measurement of blood pressure in the upper half of the body (MAPproximal), transpulmonary single indicator thermodilution-cardiac output (CO), and global end-diastolic volume (GEDV), a well-accepted marker of cardiac preload [17], as well as in the V. jugularis dextra for measurement of central venous pressure (CVP) and drug infusion. Via femoral cut-down, catheter sheaths were introduced into the Aa. femorales sinistra and dextra for distal blood pressure recording (MAPdistal) and placement of inflatable balloon catheters. Adapting a technique previously published by other authors [18], one catheter was placed directly above the aortic trifurcation, the other one directly downstream of the A. subclavia sinistra, the correct position of which was manually controlled via a left-sided thoracotomy. This approach was chosen to prevent any perfusion of the spinal cord via collateral flow distal to the proximal balloon [19], which could result from variable bifurcation of the A. radicularis magna anterior [20]. The intra-aortic balloon occlusion was used to avoid mechanical injury related to clamp placement and release per se [21]. A minilaparotomy was performed to place a catheter into the bladder for urine sampling.
Measurements and calculations
The following hemodynamic parameters were recorded continuously: heart rate, MAPproximal, MAPdistal, CO, CVP, and GEDV. Intermittent arterial blood samples were analyzed for blood gases, acid–base status, hemoglobin content, and O2 saturation. Spinal cord function was evaluated by motor evoked potentials (MEP) as described previously [4, 22, 23]. Three electrodes were inserted into the scalp and one into the soft palate to apply electric impulses (Digitimer Ltd., MultiPulse Stimulator D185 mark IIa) to the motor cortex. To quantify MEP, electrodes were inserted in the muscles of the limbs to measure the neuronal potential (ExcelTech Ltd., ExlTek Neuromax 1004). After electric stimulation of the cerebral motor cortex, the neural responses of the upper and the lower limbs were recorded. Decrease of more than 75% of the MEP amplitude was accepted as an indication of ischemic spinal cord dysfunction [22, 23]. MEP signal disappeared within 5 min in all animals as a sign of sufficient aortic occlusion. Similar to previous studies [24], MEP showed marked interindividual variability. Therefore, MEP data are presented both in µV [24] as well as in percentage of pre-ischemic values [22, 23]. In addition, spinal cord function was clinically evaluated by observing the movements of the upper and lower limbs in response to claw clamping during temporarily reduced anesthesia. The muscular response was classified as follows: 0 = no movement, 1 = muscular movement, 2 = joint movement, 3 = normal movement; a score of 4 was attributed if spontaneous movement was present even without stimulation by claw clamping.
Serum 8-epiprostaglandin F2 (8-isoprostane), interleukin (IL)-6, and tumor necrosis factor (TNF)-α levels were determined using commercial immunoassay kits as described previously [25, 26]. Data reported are normalized for protein content to correct for dilution effects resulting from volume resuscitation [25, 26]. Post mortem spinal cord samples were immediately harvested from the thoracic and lumbar regions and processed for pathohistological examination using hematoxylin and eosin (HE) and Nissl staining as well as terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL) as described in detail previously [26, 27]. A qualified neuropathologist blinded to group assignment evaluated all spinal cord sections. Complete cross-sections from the thoracic and lumber regions were evaluated. Evidence of apoptosis was accepted only if nuclear staining was considered positive. Immunohistochemical detection of cleaved caspase-3 was performed using primary antibody (cleaved caspase-3, Cell Signaling) detection by the alkaline phosphatase–anti-alkaline phosphatase complex (APAAP) method and visualization with a red chromogen (Dako APAAP REAL™, Dako Corp.) followed by counterstaining with Mayer’s hematoxylin. Slides were visualized quantified for both intensity and percent immunoreactive regions using AxioVision (release 4.8) software (Zeiss, Jena, Germany).
Experimental protocol
Twenty-seven domestic pigs (German landrace) with median (range) body weight of 49 (3–66) kg of either sex were used. The sample size was calculated based on the assumption that no neurological recovery would occur in the vehicle group [4], whereas at least 50% of the animals treated with cEPO-FC or rhEPO, respectively, would present muscle response score ≥3 (p < 0.05, power 80%). Animals were randomly assigned to receive either rhEPO [body weight 50 (36–66) kg, male/female n = 3/n = 6], cEPO-FC [body weight 48 (45–53) kg, male/female n = 5/n = 4] or vehicle [body weight 49 (44–61) kg, male/female n = 5/n = 4]. Two doses of iv rhEPO (5,000 IU kg−1 ≈ 50 μg kg−1) or cEPO-FC (50 μg kg−1) each were infused twice, i.e., over 30 min immediately before aortic occlusion and during the first 4 h of reperfusion. This EPO dose was chosen because it had yielded marked neuroprotection in models of spinal cord injury and cerebral I/R injury [9, 12, 28, 29]. Furthermore, a similar dose (40 μg kg−1) of both rhEPO and cEPO was neuroprotective in a rat model of spinal cord hemisection [30]. The cEPO-FC molecule used is a fusion protein comprising two rhEPO molecules connected with the Fc domain of a human antibody IgG1 [14]. Thereafter, this whole complex is carbamylated until no erythropoietic potency remains. The same amount of protein was administered in the cEPO-FC and rhEPO groups, but taking into account the steric molecular structure and the molecular weight of cEPO-FC, the number of EPO subunits administered in the cEPO-FC group was approximately 44% of that in the rhEPO group. Baseline data were collected, and immediately after the first rhEPO or cEPO dose, the balloon catheters were inflated until disappearance of MAPdistal. To closely mimic the clinical scenario and based on our previous studies [4, 15, 26, 27, 31], continuous iv nitroglycerin (1.7 mg min−1), esmolol (16.5 mg min−1), and adenosine-5′-triphosphate (ATP, 2–10 mg min−1) were infused and adjusted to maintain MAPproximal at 80–120% of the baseline value. To ensure constant fluid administration, animals received 10 mL kg−1 h−1 Ringer’s solution throughout the whole experiment. To optimize preload at comparable central venous pressures, 1,000 mL hydroxyethyl starch was infused during aortic occlusion. The 30-min aortic occlusion was chosen because other authors reported large spinal cord infarction over several segments in pigs after a clamping period of 60 min or longer [23]. Furthermore, 45 min of ischemia had resulted in marked ischemic neuron damage (25–40% of all thoracic motor neurons) in the spinal cord without any functional recovery at 8 h of reperfusion in our previous study [4]. During reperfusion, continuous iv noradrenaline was titrated to keep MAPdistal at the level prior to aortic occlusion. Additional data were collected at 1, 2, 4, and 10 h of reperfusion. Blood samples for measurement of 8-isoprostane, IL-6, and TNF-α levels were taken at 2 and 10 h of reperfusion, because we had observed the most pronounced effects on these parameters in previous studies on porcine aortic occlusion-induced I/R injury [26, 31]. Thereafter, the animals were sacrificed under deep anesthesia with an additional dose of Na-pentobarbitone and intravenous KCl.
Statistical analysis
All data are presented as median (range). After exclusion of normal distribution using the Kolmogorov–Smirnov test, data within groups were analyzed using Friedman repeated-measures analysis of variance on ranks and a subsequent post hoc multiple-comparison procedure (Dunn′s method). Differences between treatment groups at identical time points were analyzed with Kruskal–Wallis analysis of variance on ranks followed by a post hoc Dunn test.
Results
Table 1 summarizes the systemic hemodynamics and gas exchange. According to the protocol, MAPproximal was maintained at 80–120% of the baseline value during aortic occlusion without any intergroup difference [MAPproximal at 15 min of aortic occlusion: vehicle group: 90 (68; 101), cEPO-FC: 91 (73; 100), rhEPO: 84 (74; 97) mmHg; p = 0.302]. None of the parameters showed any significant difference between the three experimental groups at any time point of the experiment, and the total amount of noradrenaline needed to achieve the hemodynamic targets during reperfusion did not differ either [vehicle group: 162 (54; 513), cEPO-FC: 68 (30; 242), rhEPO: 82 (44; 255) µg kg−1; p = 0.174]. Table 2 summarizes the results of the blood 8-isoprostane, IL-6, and TNF-α measurements. Levels of 8-isoprostane, IL-6, and TNF-α were significantly increased at the end of the reperfusion period in all three groups, but while there was no significant intergroup difference in the cytokine blood levels, 8-isoprostane was significantly lower in both the cEPO-FC and rhEPO groups, respectively (p < 0.002 versus vehicle group).
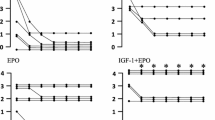
The results of the MEP measurements are shown in Table 3 and Fig. 1. Absolute MEP amplitudes showed a large interindividual range prior to ischemia (Table 3), and were significantly depressed during reperfusion. The depression of the MEP amplitude was significantly attenuated, however, in the cEPO-FC and rhEPO groups (Table 3). Figure 1 shows the MEP amplitude in percentage of pre-ischemic value and demonstrates that cEPO-FC was associated with improved recovery of spinal cord function at the end of the experiment [vehicle group: 0 (0; 32) %; cEPO-FC 63 (5; 169) %; rhEPO 10 (0; 109) %; p < 0.05 cEPO-FC versus vehicle group and rhEPO]. The intensity of the lower limb reflexes (Fig. 2) as assessed by the muscular response score to stimulation with claw clamping comparably recovered in the cEPO-FC and rhEPO groups [vehicle group: 0 (0; 4); cEPO: 4 (2; 4); rhEPO: 4 (0; 4); p < 0.001 versus vehicle group at 10 h of reperfusion]. The upper limb response remained normal in all animals throughout the whole observation period.
Amplitudes of the motor evoked potentials (MEP) (in percentage of pre-ischemia value) in the vehicle (light grey box plots), cEPO-FC (dark grey box plots), and rhEPO (open box plots) groups at 10 h of reperfusion. All data are median (quartiles, range), n = 9 in each group. § p < 0.05 versus vehicle group, # p < 0.05 versus rhEPO
Score of the lower limb muscular response to stimulation with claw clamping before, and at 10 h of, reperfusion in the vehicle (light-grey box plots), cEPO-FC (dark-grey box plots), and rhEPO (open box plots) groups. A score of 4 was attributed when spontaneous movement was present even without claw clamping. All data are median (quartiles, range), n = 9 in each group. § p < 0.05 versus vehicle group
The histological evaluation showed that attenuation of postischemic neurological dysfunction coincided with significantly less pronounced neuronal damage. Hematoxylin eosin and Nissl staining showed cell swelling and cytoplasmatic vacuolization in both the lumbar and the thoracic spinal cord slices, indicating marked ischemic spinal cord injury (Fig. 3a–c). Infiltration of mononuclear cells was present in a few animals only, without intergroup difference. Nissl staining allowed quantification of the number of neurons with ischemic damage: both cEPO-FC and rhEPO comparably attenuated neuronal injury in the spinal cord (Fig. 3d), and this protection was similar in the thoracic [vehicle group: 27 (11; 47); cEPO-FC: 8 (2; 15); rhEPO: 5 (4; 11)% Nissl-positive cells as fraction of all cells; p < 0.05 versus vehicle group] and the lumbar regions [vehicle group: 26 (16; 41); cEPO-FC: 7 (3; 22); rhEPO 8 (0; 19) % Nissl-positive cells as fraction of all cells; p < 0.05 versus vehicle group]. Post mortem immune in situ hybridization of spinal cord cross-sections (TUNEL) did not reveal significant apoptosis activity in either groups [thoracic region: vehicle group 0 (0; 4), cEPO-FC 0 (0; 1), rhEPO 0 (0; 19); lumbar region: vehicle group 0 (0; 0), cEPO-FC 0 (0; 1), rhEPO 0 (0; 1) TUNEL-positive cells], and there was no significant intergroup difference. Positive immunohistochemical staining for activated caspase-3 was nearly completely absent well.
Examples of hematoxylin and eosin (a, ×10 magnification) and Nissl (b and c, ×20 magnification) staining of a spinal cord cross-section as well as quantification of Nissl staining (d). The histological examples show eosinophilic ganglion cell necrosis (a), cell swelling and cytoplasmatic vacuolization (a, b) as markers of ischemic spinal cord injury, and infiltration of mononuclear cells (c). Quantification of the Nissl staining (d) shows the total number of Nissl-positive neurons as percentage of the total neuron count after 10 h of reperfusion in the vehicle (light-grey box plots), cEPO-FC (dark-grey box plots), and rhEPO (open box plots) groups. All data are median (quartiles, range), n = 9 in each group, § p < 0.05 versus vehicle group
Discussion
The aim of the present study is to test the hypothesis that cEPO-FC compares favorably with rhEPO in a clinically relevant porcine model of thoracic aortic balloon occlusion-induced spinal cord I/R injury. The major findings were (1) that cEPO-FC more effectively restored motor evoked potentials than rhEPO, whereas (2) the loss of spinal cord reflexes was comparably improved by the two drugs. The protective effect on neurological function coincided with a (3) similar attenuation of ischemic neuron damage and (4) markers of lipid peroxidation.
While the score of the muscular response to claw clamping during reperfusion was comparably improved in the two treatment groups, cEPO-FC more efficiently restored motor evoked potentials than did rhEPO in our experiment. A different effect on the systemic inflammatory response and/or oxidative stress between these two compounds can most likely be ruled out: in contrast to the data recently reported by Nijboer et al. [13], neither cEPO-FC nor rhEPO affected the rise in IL-6 and TNF-α levels, and the attenuation of the rise in blood isoprostane levels was comparable. Furthermore, rhEPO and cEPO-FC treatment resulted in similar protection against neuronal damage, and infiltration of inflammatory cells into the spinal cord did not show any intergroup difference either.
Both cEPO-FC and rhEPO attenuated ischemic neuronal damage in the spinal cord, which coincided with improved neurological function. These findings are in contrast with our previous report demonstrating that rhEPO allowed reduction of spinal cord tissue damage without, however, any beneficial effect on neurological function [4]. The shorter ischemia period (30 min of aortic occlusion in the present versus 45 min in the previous study) resulted in much less pronounced overall neuronal injury damage and is most likely responsible for this discrepancy; in fact, in the previous study even the rhEPO-treated animals presented with 25% and 30% Nissl-positive neurons in the thoracic and lumbar spinal cord, respectively. In the present study such a high number of Nissl-positive neurons was only found in the vehicle group. These animals, however, did not show any neurological recovery either. It is noteworthy in this context that immunohistochemical staining for caspase-3 and TUNEL-positive glia cells were virtually absent, which is in contrast with previous reports from our own group [4] and by other authors [32] on porcine spinal cord I/R and traumatic injury, respectively. Again, the shorter ischemia may assume importance: in the previous study we found TUNEL-positive cells in both the thoracic and lumbar regions after 45 min of spinal cord ischemia, whereas in another experiment only minor positive TUNEL staining was present in the spinal cord after 30 min of aortic occlusion [26]. The time lag between tissue injury and organ sampling is also crucial for detection of postinjury apoptosis in the spinal cord: while caspase-3-positive cells were reported 28 days after spinal cord trauma [32], we hardly found any TUNEL-positive cells 4 h after spinal cord ischemia [27]. Controversial data are available on the presence of neuronal apoptosis after spinal cord I/R injury per se: while TUNEL-positive staining was reported in rats [33] and rabbits [2, 34], other authors did not find any TUNEL-positive neurons in swine even at 120 h of reperfusion [18]. Finally, apoptotic cell death was reported to assume importance in immature neurons only [35]: we studied swine aged 18–24 weeks, and to the best of our knowledge, neuronal apoptosis was only described in neonatal and infant piglets with maximum age of 4–5 weeks [32, 36].
The cytoprotective effects of rhEPO and its nonhematopoietic derivatives are attributed to various mechanisms [37], i.e., anti-inflammatory [13, 28], anti-oxidative [13, 29, 38], and anti-apoptotic [8, 9, 28, 38] properties. In our experiments, neither cEPO-FC nor rhEPO affected the I/R-induced increase in blood cytokine levels or the mononuclear cell infiltration, nor was there evidence of apoptosis in any of the groups. The significantly lower levels of isoprostane, a well-accepted marker of lipid peroxidation [39], may suggest that cEPO-FC and rhEPO probably exerted their neuroprotective properties due to attenuation of oxidative stress. Clearly, this systemic anti-oxidative property of a systemically administered drug does not necessarily explain a localized protective effect within the spinal cord: there was no significant relation between the percentage recovery of MEP amplitude or the lower limb muscle response score, respectively, and blood isoprostane levels (coefficient of correlation r = −0.19, p = 0.346, and r = −0.27, p = 0.171, respectively). Nevertheless, our findings are in good agreement with previous data on both rhEPO- and cEPO-induced attenuation of oxidative stress in rodent models of I/R injury [13, 38]. Finally, Siems et al. [40] also reported a reduction of blood isoprostane levels in anemic patients treated with rhEPO.
Limitations of the study
First, during aortic occlusion, we continuously infused nitroglycerin, esmolol, and ATP in order to control blood pressure. It could be argued that this pharmacological intervention influenced our findings, given the beneficial effect of ATP infusion in experimental I/R injury [41]. It must be emphasized, however, that the infusion rates of these drugs were strictly identical in the three groups and that they were only administered during the 30 min of aortic occlusion. Therefore, it is unlikely that they produced major effects that could be evidenced as late as at 10 h of reperfusion. Moreover, a second major limitation may be related to the duration of the reperfusion period. In fact, other authors reported evaluation of neurological function and spinal cord histology after 24–120 h of reperfusion [18, 42]. These studies have demonstrated that both neurological outcome and tissue damage may further worsen after the first 10 h of reperfusion [18]. Consequently, we may have missed more pronounced differences between cEPO-FC and rhEPO, respectively, than those observed for the motor evoked potentials.
In summary, in a porcine model of aortic occlusion-induced spinal cord I/R injury we demonstrated that the newly developed cEPO-FC and rhEPO comparably attenuated neuronal damage and restored neurological function. The neuroprotective properties coincided with similar attenuation of lipid peroxidation, whereas anti-inflammatory and anti–apoptotic effects most likely did not assume major importance.
References
Gelman S (1995) The pathophysiology of aortic cross-clamping and unclamping. Anesthesiology 82:1026–1060
Celik M, Gökmen N, Erbayraktar S, Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E, Cerami A, Brines M (2002) Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA 99:2258–2263
Sönmez A, Kabakçi B, Vardar E, Gürel D, Sönmez U, Orhan YT, Açikel U, Gökmen N (2007) Erythropoietin attenuates neuronal injury and potentiates the expression of pCREB in anterior horn after transient spinal cord ischemia in rats. Surg Neurol 68:297–303
Simon F, Scheuerle A, Calzia E, Bassi G, Oter S, Duy CN, Kick J, Brückner UB, Radermacher P, Schelzig H (2008) Erythropoietin during porcine aortic balloon occlusion-induced ischemia/reperfusion injury. Crit Care Med 36:2143–2150
Ehrenreich H, Weissenborn K, Prange H, Schneider D, Weimar C, Wartenberg K, Schellinger PD, Bohn M, Becker H, Wegrzyn M, Jähnig P, Herrmann M, Knauth M, Bähr M, Heide W, Wagner A, Schwab S, Reichmann H, Schwendemann G, Dengler R, Kastrup A, Bartels C, Stroke Trial Group EPO (2010) Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke 40:647–656
Nagai T, Akizawa T, Kohjiro S, Koiwa F, Nabeshima K, Niikura K, Kino K, Kanamori N, Kinugasa E, Ideura T (1996) rHuEPO enhances the production of plasminogen activator inhibitor-1 in cultured endothelial cells. Kidney Int 50:102–107
Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, Latini R, Xie QW, Smart J, Su-Rick CJ, Pobre E, Diaz D, Gomez D, Hand C, Coleman T, Cerami A (2004) Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroceptor. Proc Natl Acad Sci USA 1:14907–14912
Coleman TR, Westenfelder C, Tögel FE, Yang Y, Hu Z, Swenson L, Leuvenink HG, Ploeg RJ, d Uscio LV, Katusic ZS, Ghezzi P, Zanetti A, Kaushansky K, Fox NE, Cerami A, Brines M (2006) Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different procoagulant and vasoactive activities. Proc Natl Acad Sci USA 103:5965–5970
Brines M, Cerami A (2008) Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Int Med 264:405–432
Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-Hand C, Xie QW, Coleman T, Cerami A, Brines M (2004) Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305:239–242
Adembri C, Massagrande A, Tani A, Miranda M, Margheri M, De Gaudio R, Pellegrini-Giampietro DE (2008) Carbamylated erythropoietin is neuroprotective in an experimental model of traumatic brain injury. Crit Care Med 36:975–978
Lapchak PA, Kirkeby A, Zivin JA, Sager TN (2008) Therapeutic window for nonerythropoietic carbamylated-erythropoietin to improve motor function following multiple infarct ischemic strokes in New Zealand white rabbits. Brain Res 1238:208–214
Nijboer WN, Ottens PJ, van Dijk A, van Goor H, Ploeg RJ, Leuvenink HGD (2010) Donor pretreatment with carbamylated erythropoietin in a brain death model reduces inflammation more effectively than erythropoietin while preserving renal function. Crit Care Med 38:1155–1161
Schriebl K, Trummer E, Lattenmayer C, Weik R, Kunert R, Müller D, Katinger H, Vorauer-Uhl K (2006) Biochemical characterization of rhEPO-Fc fusion protein expressed in CHO cells. Protein Expr Purif 49:265–275
Simon F, Giudici R, Duy CN, Schelzig H, Oter S, Gröger M, Wachter U, Vogt J, Speit G, Szabó C, Radermacher P, Calzia E (2008) Hemodynamic and metabolic effects of hydrogen sulfide during porcine ischemia/reperfusion injury. Shock 30:359–364
Kuriyama T, Latham LP, Horwitz LD, Reeves JT, Wagner WW Jr (1984) Role of collateral ventilation in ventilation-perfusion balance. J Appl Physiol 56:1500–1506
Nirmalan M, Willard TM, Edwards DJ, Little RA, Dark PM (2005) Estimation of errors in determining intrathoracic blood volume using the single transpulmonary thermal dilution technique in hypovolemic shock. Anesthesiology 103:805–812
Papakostas JC, Matsagas MI, Toumpoulis IK, Malamou-Mitsi VD, Pappa LS, Gkrepi C, Anagnostopoulos CE, Kappas AM (2006) Evolution of spinal cord injury in a porcine model of prolonged aortic occlusion. J Surg Res 133:159–166
Blaisdell FW, Cooley DA (1962) The mechanism of paraplegia after temporary thoracic aortic occlusion and its relationship to spinal fluid pressure. Surgery 51:351–355
Wadouh F, Lindemann EM, Arndt CF, Hetzer R, Borst HG (1984) The arteria radicularis magna anterior as a decisive factor influencing spinal cord damage during aortic occlusion. J Thorac Cardiovasc Surg 88:1–10
Weigang E, Luehr M, von Samson P, Hartert M, Goebel H, Wetzig M, Bernard V, Siegenthaler MP, Beyersdorf F (2005) Development of a special balloon occlusion device top prevent adverse events in high-risk patients during open aortic surgery. Eur Surg Res 37:204–209
Meylaerts SA, De Haan P, Kalkman CJ, Lips J, De Mol BA, Jacobs MJ (1999) The influence of regional spinal cord hypothermia on transcranial myogenic motor-evoked potential monitoring and the efficacy of spinal cord ischemia detection. J Thorac Cardiovasc Surg 118:1038–1045
Meylaerts SA, De Haan P, Kalkman CJ, Jaspers J, Vanicky I, Jacobs MJ (2000) Prevention of paraplegia in pigs by selective segmental artery perfusion during aortic crossclamping. J Vasc Surg 32:160–170
Lips J, de Haan P, Bouma GJ, Holman R, van Dongen E, Kalkman CJ (2005) Continuous monitoring of cerebrospinal fluid oxygen tension in relation to motor evoked potentials during spinal cord ischemia in pigs. Anesthesiology 102:340–345
Hauser B, Kick J, Iványi Z, Asfar P, Ehrmann U, Muth CM, Albicini M, Wachter U, Vogt J, Bauer M, Brückner UB, Radermacher P, Bracht H (2006) Effects of 15-deoxy-Δ12, 14-prostaglandin-J2 during hyperdynamic porcine endotoxemia. Intensive Care Med 3:759–765
Kick J, Hauser B, Bracht H, Albicini M, Oter S, Simon F, Ehrmann U, Garrel C, Sträter J, Brückner UB, Leverve XM, Schelzig H, Speit G, Radermacher P, Muth CM (2007) Effects of a cantaloupe melon extract/wheat gliadin biopolymer during aortic cross-clamping. Intensive Care Med 33:694–702
Maier C, Scheuerle A, Hauser B, Schelzig H, Szabó C, Radermacher P, Kick J (2007) The selective PARP-1 inhibitor INO1001 reduces spinal cord injury during porcine aortic cross-clamping-induced ischemia/reperfusion injury. Intensive Care Med 33:845–850
Villa P, van Beek J, Larsen AK, Gerwien J, Christensen S, Cerami A, Brines M, Leist M, Ghezzi P, Torup L (2007) Reduced functional deficits, neuroinflammation, and secondary tissue damage after treatment of stroke by nonerythropoietic erythropoietin derivatives. J Cerebr Blood Flow Metab 27:552–563
Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E (2004) Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev 27:113–120
King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT (2007) Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci 26:90–100
Simon F, Scheuerle A, Gröger M, Stahl B, Wachter U, Vogt J, Speit G, Hauser B, Möller P, Calzia E, Szabó C, Schelzig H, Georgieff M, Radermacher P, Wagner F (2011) Effects of intravenous sulfide during porcine aortic occlusion-induced kidney ischemia/reperfusion injury. Shock 35:156–163
Kuluz J, Samdani A, Benglis D, Gonzalez-Brito M, Solano JP, Ramirez MA, Luqman A, De los Santos R, Hutchinson D, Nares M, Padgett K, He D, Huang T, Levi A, Betz R, Dietrich D (2010) Pediatric spinal cord injury in infant piglets: description of a new large animal model and review of the literature. J Spinal Cord Med 33:43–57
Lang-Lazdunski L, Heurteaux C, Mignon A, Mantz J, Widmann C, Desmonts J, Lazdunski M (2000) Ischemic spinal cord injury induced by aortic cross-clamping: prevention by riluzole. Eur J Cardiothorac Surg 18:174–181
Wang Q, Ding Q, Zhou Y, Gou X, Hou L, Chen S, Zhu Z, Xiong L (2009) Ethyl pyruvate attenuates spinal cord ischemic injury with a wide therapeutic window through inhibiting high-mobility group box 1 release in rabbits. Anesthesiology 110:1279–1286
Hu BR, Liu CL, Ouyang Y, Blomgren K, Siesjö BK (2000) Involvement of caspase-3 in cell death after hypoxia-ischemia declines during brain maturation. J Cereb Blood Flow Metab 20:1550–1556
Kuluz JW, Prado R, He D, Zhao W, Dietrich WD, Watson B (2007) New pediatric model of ischemic stroke in infant piglets by photothrombosis: acute changes in cerebral blood flow, microvasculature, and early histopathology. Stroke 38:1932–1937
Maiese K, Li F, Chong ZZ (2005) New avenues of exploration for erythropoietin. JAMA 293:90–95
Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, Sollott SJ, Lakatta EG, Talan MI (2006) Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther 316:999–1005
Basu S, Eriksson M (1998) Oxidative injury and survival during endotoxemia. FEBS Lett 438:159–160
Siems W, Quast S, Carluccio F, Wiswedel I, Hirsch D, Augustin W, Kraemer K, Hampl H, Sommerburg O (2003) Oxidative stress in cardio renal anemia syndrome: correlations and therapeutic possibilities. Clin Nephrol 60 Suppl 1:S22–S30
Nalos M, Asfar P, Ichai C, Radermacher P, Leverve XM, Fröba G (2003) Adenosinetriphosphate-magnesium chloride: relevance for intensive care. Intensive Care Med 29:10–18
Maharajh GS, Pascoe EA, Halliday WC, Grocott HP, Thiessen DB, Girling LG, Cheang MS, Mutch WA (1996) Neurological outcome in a porcine model of descending thoracic aortic surgery. Left atrial–femoral artery bypass versus clamp/repair. Stroke 27:2095–2101
Acknowledgment
This study was supported by the Deutsche Forschungsgemeinschaft (DFG Sche 899/2-3). Carbamylated erythropoietin fusion protein (cEPO-FC) was kindly provided by Polymun Scientific GmbH, Vienna, Austria. The authors are indebted to Andrea Söll and Tanja Schulz for skillful assistance.
Conflicts of interest
B. Vcelar is an employee responsible for preclinical research at Polymun Scientific GmbH (Vienna, Austria), a company involved in the commercial development of cEPO-FC, but holds no equity in that company nor related to the molecules investigated. The other authors declare that they have no competing interests at all.
Author information
Authors and Affiliations
Corresponding author
Additional information
Florian Simon, Angelika Scheuerle, and Michael Gröger contributed equally to this study.
This article is discussed in the editorial available at: doi:10.1007/s00134-011-2305-2.
Rights and permissions
About this article
Cite this article
Simon, F., Scheuerle, A., Gröger, M. et al. Comparison of carbamylated erythropoietin-FC fusion protein and recombinant human erythropoietin during porcine aortic balloon occlusion-induced spinal cord ischemia/reperfusion injury. Intensive Care Med 37, 1525–1533 (2011). https://doi.org/10.1007/s00134-011-2303-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-011-2303-4