Abstract
Purpose
Extracorporeal membrane oxygenation (ECMO) can support oxygenation and carbon dioxide elimination in severe lung failure. Usually it is accompanied by controlled mechanical ventilation. Neurally adjusted ventilatory assist (NAVA) is a new mode of ventilation triggered by the diaphragmatic electrical activity and controlled by the patient’s respiratory centre, which may allow a close interaction between ventilation and extracorporeal perfusion. This pilot study intended to measure the physiologic ventilatory response in patients with severe lung failure treated with ECMO and NAVA. We hypothesized that the combination of both methods could automatically provide a protective ventilation with optimized blood gases.
Methods
We report a case series of six patients treated with ECMO for severe lung failure. In the recovery phase of the disease, patients were ventilated with NAVA and ventilatory response and gas exchange parameters were measured under different sweep gas flows and temporarily inactivated ECMO.
Results
Tidal volumes on ECMO ranged between 2 and 5 ml/kg predicted body weight and increased up to 8 ml/kg with inactivated ECMO. Peak inspiratory pressure reached 19–29 cmH2O with active, and 21–45 cmH2O with inactivated ECMO. Ventilatory response to decreased sweep gas flow was rapid, and patients immediately regulated PaCO2 tightly towards a physiological pH value. Increase in minute ventilation was a result of increased breathing frequency and tidal volumes, and protective ventilation was only abandoned if pH control was not achieved.
Conclusions
With NAVA ventilatory response to decreased ECMO sweep gas flow was rapid, and patients immediately regulated PaCO2 tightly towards a physiological pH value. Therefore, combination of NAVA and ECMO may permit a closed-loop ventilation with automated protective ventilation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mechanical ventilation with low tidal volumes (6 ml/kg predicted body weight) is well established as one of the essential treatments for patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [1–4]. Despite relevant improvements in therapy in recent years, there is still a high mortality rate among patients with severe lung failure [5, 6]. Extracorporeal membrane oxygenation was shown to be effective in the treatment of ARDS and remains a rescue therapy for patients with severe lung failure [7–13]. New miniaturized systems reduce haemorrhagic and thrombembolic complications due to important technical improvements with a lower surface area and a diminished need for anticoagulation.
However, combining ECMO with conventional protective ventilation often requires deep sedation with disjunction of extracorporeal perfusion and ventilation. Both systems for gas transfer, extracorporeal lung support and mechanical ventilation, are independent and have to be readjusted repeatedly. Despite close supervision, hypocapnia and unphysiological pH levels are commonly observed due to the high carbon dioxide elimination capacity of extracorporeal devices. Furthermore, prolonged controlled ventilation can result in rapid atrophy of the diaphragm, which has been denoted ventilator-induced diaphragmatic dysfunction (VIDD) [14, 15]. Therefore early spontaneous breathing could reduce VIDD and decreased sedation allows early mobilisation important for better functional outcomes [16].
Connecting vv-ECMO with assisted spontaneous breathing may prove to be advantageous, especially in regard to a physiological acid–base balance and possibly prevention of VIDD. Neurally adjusted ventilatory assist (NAVA) is a new mode of assisted closed-loop ventilation, which is triggered by the electrical activity of the diaphragm (EAdi) [17–22]. Pressure support is amplified proportionally to the EAdi signal, and assisted ventilation is controlled by the patient’s respiratory centre. The hypothesis of the current study was that combining NAVA and vv-ECMO results in normalisation of blood gases and pH and provides a highly protective ventilation in patients with severe ARDS. Feasibility of coupling both methods was proven in clinical practice and physiologic response was determined.
Materials and methods
Study population
Six adult patients with severe respiratory failure due to bilateral pneumonia treated with veno-venous ECMO and NAVA were included in this study. All patients had been transferred from external hospitals, because severe ARDS, defined according to the criteria of the American–European Conference on ARDS [23], had not been stabilized on conventional ventilation. In two patients too unstable for transfer, ECMO was started by our mobile team before transportation. In the other patients extracorporeal veno-venous lung support was initiated because, despite continuing efforts in our centre to improve gas exchange with optimised protective ventilation and prone positioning, the PaO2/FiO2 ratio remained at less than 100 mmHg and/or severe respiratory acidosis could not be controlled. After implementation of ECMO, invasiveness of mechanical ventilation was reduced to avoid further ventilator-induced lung injury. Preset goals for oxygenation were a PaO2 of greater than 65 mmHg and PaCO2 was adjusted to achieve a normal arterial pH level.
Extracorporeal lung support
The extracorporeal system consists of two venous cannulas, a centrifugal pump and a membrane oxygenator (Maquet Cardiopulmonary AG, Hirrlingen, Germany). Detailed technique is given in the online data supplement.
Neurally adjusted ventilatory assist (NAVA)
All patients were ventilated with the Servo-I ventilator including the NAVA option (Maquet Critical Care, Rastatt, Germany). During NAVA the EAdi signal is processed as described previously [17, 24], and pressure support is applied proportionally to the EAdi signal. The EAdi signal in microvolts is multiplied by a gain factor called NAVA level and thereby transposed into pressure support. The NAVA level was individually titrated. In brief, patients were ventilated with pressure support ventilation (PSV) aiming at a physiological breathing pattern, optimal blood gases and no obvious stress. Subsequently the NAVA level was individually set so that peak pressure and pressure delivery were comparable to pressure support in PSV with use of the NAVA preview tool, which is integrated into the software. NAVA was established at least 1 day before study entry and patients were ventilated further on with NAVA as the preferred ventilatory mode. Patients were sedated solely with short-acting agents (propofol and remifentanil or sufentanil) with an intended RASS score of −3 to −1. [25]. During the measuring period patients were not actively moved, a calm environment was established to avoid arousals, and no change in sedation was allowed.
Data acquisition and analysis
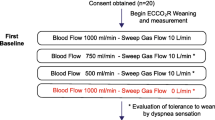
Data were collected prospectively. After several hours in stable condition on a preset NAVA level, blood gas analysis and ventilatory parameters were documented at five consecutive times, each data set for a period of 30 min: at baseline, after reduction of ECMO sweep gas flow to 50%, again after temporarily pausing sweep gas flow, after increase of FiO2 to achieve a saturation at or above 95% and finally again at baseline settings. Further information is given in the “Electronic supplementary material”.
Approval for this study was gained from the Ethics Committee of the University of Regensburg. Informed consent was obtained from the legal representatives of the patients, and agreement for publication of data from the patients. All patients were under constant supervision of an experienced intensivist; no relevant side effects occurred.
Variables are reported as mean with a 95% confidence interval. Non-parametric ANOVA test was used for statistical analysis. A p value of less than 0.05 was considered statistically significant. For statistical analysis, we used GraphPad Prism 4 for Macintosh computer (La Jolla, CA 92037 USA). Data were acquired with the Ventilation-Record-Card SERVO-i Vers. 1.3 and in parallel with SERVO-i-RCR Version 2.3 (Maquet, Critical Care, Rastatt, Germany).
Results
Patients’ characteristics before and after 1 day on ECMO are summarized in Table 1. Individual diagnoses and baseline characteristics are documented in Table S1 in the “Electronic supplementary material”. All patients had a favourable outcome, five survived to discharge; the sixth was transferred for successful lung transplant.
PaO2, PaCO2 and pH
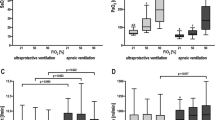
Oxygen transfer of the ECMO, calculated by multiplying the difference in oxygen content before and after the oxygenator by the blood flow, averaged 112 ± 37 ml/min. ECMO blood flow before and during measurements was 1.9 ± 0.36 l/min. At baseline all patients were in a stable state of oxygenation. Reducing sweep gas flow of the ECMO to 50% at unchanged blood flow resulted in most patients in an increase of PaO2 (Fig. 1). At a sweep gas flow of 0 l/min, which implies that extracorporeal gas transfer is stopped temporarily, all patients had an expected decrease of PaO2, which was controlled by the consecutive increase of FiO2 (Fig. 1; p < 0.05).
PaO2 und PaCO2 (dashed line) with different ECMO/NAVA settings (patient A–F). At baseline all patients were in a stable state of oxygenation. Reducing sweep gas flow of the ECMO to 50% at unchanged blood flow results in most patients in an increase of PaO2. At a sweep gas flow of 0 l/min, which implies that extracorporeal gas transfer is stopped temporarily, all patients had an expected decrease of PaO2 (p < 0.05), which was controlled by the consecutive increase of FiO2
The estimated carbon dioxide elimination capacity of the ECMO was calculated with 161 ± 30 ml/min. Decreasing and turning off the sweep gas flow was compensated at least partially by all patients but one. Stable PaCO2 and pH values were observed because of ventilatory compensation (see below). Patient E with end-stage cystic fibrosis could not sufficiently increase pulmonary carbon dioxide elimination (Fig. 1) mainly caused by a low compliance of the respiratory system (11 ml/cmH2O). Consecutively, pH in this patient dropped considerably, whereas control within the physiologic range was maintained in the other patients (Fig. 2). Returning to baseline by turning on the sweep gas flow again was followed in most cases by a drop of PaCO2 and an increase of pH.
Electrical diaphragmatic activity and peak inspiratory pressure
Stepwise reducing and turning off the ECMO sweep gas flow led in all cases to an increased electrical diaphragmatic activity (EAdi peak). Values ranged in mean from 2 to 32 μV with active sweep gas flow and from 4 to 47 μV with inactivated gas flow (Fig. 3). Proportional to the EAdi peak signal, peak inspiratory pressure (PIP) ranged in mean from 19 to 29 cmH2O with active ECMO and from 21 to 45 cmH2O with inactivated ECMO (p < 0.05, Fig. S1). With a preset PEEP level of 10 cmH2O or more, most patients did not increase the mean PIP above 30 cmH2O. Only patient E tolerated a PIP of more than 40 cmH2O with a very high EAdi peak signal in the futile attempt to control hypercapnia.
Tidal volume (V T) and minute ventilation (V E)
Since pressure is applied proportionally to the electrical activity of the diaphragm, in the NAVA mode tidal volumes vary from breath to breath, controlled by the patient’s respiratory centre. Tidal volumes with running ECMO ranged between 2 and 5 ml/kg predicted body weight. Reducing and turning off the sweep gas flow led in all cases to an increased V T (p < 0.01, Fig. 4), in patients A, D and F to a V T above 6 ml/kg. Hyperoxygenation by increasing the FiO2 with inactive ECMO did not reduce V T (p > 0.05, Fig. 4).
Tidal volumes (V T) with running ECMO ranged between 2 and 5 ml/kg predicted body weight. Reducing and turning off the sweep gas flow led in all cases to an increased V T (p < 0.01), in patients A, D and F to a V T above 6 ml/kg. Hyperoxygenation by increasing the FiO2 with inactive ECMO does not reduce V T
Minute ventilation (V E) increased rapidly in all patients after reducing and turning off the sweep gas flow (p < 0.05, Fig. S2). Ventilation increased 2.3-fold in patient 1, 1.3-fold in patient 2, 3.4-fold in patient 3, 2.3-fold in patient 4, 1.6-fold in patient 5 and 1.1-fold in patient 6 at turned off gas flow compared to baseline (Fig. S2). The rise in V E was partly a result of increased V T, but mainly caused by an increase in breathing frequency (Fig. S3).
Discussion
The present pilot study demonstrates for the first time that a coupling of extracorporeal membrane oxygenation with neurally adjusted ventilation is feasible in clinical practice. Our results indicate that homeostasis of pH value and PaCO2 are the determining factors for spontaneously breathing patients on vv-ECMO and NAVA. Most patients automatically achieve a protective ventilation with low tidal volumes as well as peak inspiratory pressures below 30 cmH2O. This opens a so far unknown opportunity for a new form of closed-loop ventilation regulated by the patient’s respiratory centre.
Current clinical practice in patients with severe lung failure who require extracorporeal lung support usually combines vv-ECMO with controlled mechanical low-pressure, low-tidal ventilation [8, 11, 26–28]. Such a strategy requires deep sedation and may lead to a complete disjunction of extracorporeal perfusion and ventilation. Whilst controlled mechanical ventilation may be indispensable in the initial phase of severe ARDS on vv-ECMO to reliably control tidal volume and peak pressure, control of blood gases, perfusion settings and ventilatory parameters has to be tight, and regulation of oxygenation, pH level and carbon dioxide with two independent systems can be challenging. Transient overshooting is commonly observed due to the high CO2 removal capability of ECMO systems. Therefore a closed-loop system, which couples extracorporeal perfusion with ventilation could be advantageous to achieve homeostasis of blood gases. Though not possible in the very early phase of ARDS with pulmonary capillary leak syndrome, such a system should be established as soon as patients begin to recover. In the setting reported here with combined vv-ECMO and NAVA, the patient is given the opportunity to regulate ventilation by EAdi-proportional pressure support, varying breathing frequency, peak pressure and tidal volume, while the therapist only regulates extracorporeal perfusion and PEEP level. NAVA ventilation was established as the preferred ventilation mode as soon as stabilisation from the underlying disease was clinically obvious. Although it might have been possible to choose pressure support ventilation instead, NAVA offers the advantage that patients can regulate tidal volumes and that the electrical activity of the diaphragm is monitored. All patients were able to breath even on a high PEEP level with sufficient diaphragm contraction and EAdi signal. Therefore, NAVA allowed early establishment of spontaneous breathing. This may be important to prevent diaphragm atrophy, as has been described previously for controlled mechanical ventilation [14]. Early diaphragm contraction possibly is of substantial importance for the outcome of patients with severe lung failure, since assisted spontaneous breathing modes can decrease days on mechanical ventilation and stay in the intensive care unit [29–31].
The measurements documented here were conducted in the weaning period from ECMO, as otherwise it would not have been possible to turn off the sweep gas flow completely. We could demonstrate that a response of the patient’s respiratory centre followed promptly on stepwise reducing the extracorporeal support. The main determining factor for the up-regulation of ventilation was not oxygenation, measured as PaO2, but the drive for homeostasis of carbon dioxide and pH value. These results are in line with a pivotal study by Kolobow et al. [32], who could demonstrate in lambs that minute ventilation is directly coupled to extracorporeal carbon dioxide elimination. It is noteworthy that the first reduction of sweep gas flow often resulted in an increase of PaO2 (p < 0.05, Fig. 1). The observed consecutive increase in minute ventilation may lead to recruitment of previously non-ventilated areas. With paused extracorporeal support most patients were able to control pH within the physiologic range at the cost of greatly raised V E. This increase of V E was generated by a moderate increase of V T and a pronounced rise in breathing frequency. Comparable results with a strong up-regulation of the ventilatory rate in ALI have been observed in a recent study with rabbits on NAVA [33]. Hyperoxygenation by increasing FiO2 did not show a substantial influence on respiratory drive. In contrast, restarting extracorporeal support resulted in a rapid down-regulation of ventilation.
As is to be expected, ventilation was highly protective with active ECMO. All patients chose a V T well below 6 ml/kg predicted body weight (Fig. 4). Interestingly, after turning off the ECMO, V T did not increase above 8 ml/kg, and all patients apart one avoided peak inspiratory pressures above a mean of 30 cmH2O. Although tidal volumes beyond 8 ml/kg would have been feasible with NAVA, patients still chose the best possible protective ventilation. Only patient E was not able to compensate hypercapnia and increased peak pressures to more than 40 cmH2O. The further clinical course proved that this patient was dependent on ECMO support and had to be bridged until successful lung transplantation. This suggests that homeostasis of the acid–base balance is the primary goal and may be achieved at the expense of non-protective ventilation. NAVA applies pressure support proportionally to the electrical activity of the diaphragm and enables the patient to control ventilation closely.
The present study has limitations. NAVA demands a functioning respiratory centre of the patient and is not possible on deep sedation or in some patients with brain injury. Furthermore, it has never been investigated whether the feedback mechanisms necessary for neurally assisted ventilation are adequately preserved in sepsis and ARDS. Moreover, presently there is no clear recommendation how to set the best NAVA assist level. Therefore, the chosen levels are arbitrary and based on our experience with NAVA. Although NAVA ventilation was established well before the start of the study, we cannot exclude that different NAVA levels would have led to different results. By design this study was not planned to investigate outcome; whether NAVA influences survival in mechanical ventilation is unknown. The current study does not resolve the question whether NAVA facilitates weaning from extracorporeal lung support either. Therefore, further studies are needed to determine outcome and survival of patients on vv-ECMO and NAVA.
In conclusion the combination of extracorporeal lung support and NAVA is a possible and sensible complementary application of two different techniques in patients with severe lung failure. A down-regulation of extracorporeal gas transfer caused an immediate up-regulation of ventilation. Homeostasis was closely preserved, and patients tried to maintain a protective ventilation. Tidal volumes were chosen by the patient’s respiratory centre well below 6 ml/kg predicted body weight on active ECMO and did not increase above 8 ml/kg without ECMO in our patients. Even though early results are promising, further studies are necessary to closer define the role of combined NAVA and ECMO treatment in patients with severe ARDS.
Abbreviations
- NAVA:
-
Neurally adjusted ventilatory assist
- EAdi:
-
Electrical activity of the diaphragm
- ARDS:
-
Adult respiratory distress syndrome
- ECMO:
-
Extracorporeal membrane oxygenation
- VIDD:
-
Ventilator-induced diaphragmatic dysfunction
- RASS:
-
Richmond Agitation-Sedation Scale
- PIP:
-
Peak inspiratory pressure
- PEEP:
-
Positive end-expiratory pressure
References
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med 342:1301–1308
Esteban A, Ferguson ND, Meade MO, Frutos-Vivar F, Apezteguia C, Brochard L, Raymondos K, Nin N, Hurtado J, Tomicic V, Gonzalez M, Elizalde J, Nightingale P, Abroug F, Pelosi P, Arabi Y, Moreno R, Jibaja M, D’Empaire G, Sandi F, Matamis D, Montanez AM, Anzueto A (2008) Evolution of mechanical ventilation in response to clinical research. Am J Respir Crit Care Med 177:170–177
Brochard L, Rouby JJ (2009) Changing mortality in acute respiratory distress syndrome? Yes, we can! Am J Respir Crit Care Med 179:177–178
Ranieri VM, Giunta F, Suter PM, Slutsky AS (2000) Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 284:43–44
Brun-Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, Bion J, Romand JA, Villar J, Thorsteinsson A, Damas P, Armaganidis A, Lemaire F (2004) Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med 30:51–61
Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD (2005) Incidence and outcomes of acute lung injury. N Engl J Med 353:1685–1693
Lewandowski K, Rossaint R, Pappert D, Gerlach H, Slama KJ, Weidemann H, Frey DJ, Hoffmann O, Keske U, Falke KJ (1997) High survival rate in 122 ARDS patients managed according to a clinical algorithm including extracorporeal membrane oxygenation. Intensive Care Med 23:819–835
Hemmila MR, Rowe SA, Boules TN, Miskulin J, McGillicuddy JW, Schuerer DJ, Haft JW, Swaniker F, Arbabi S, Hirschl RB, Bartlett RH (2004) Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann Surg 240:595–605; discussion 605–597
Schuerer DJ, Kolovos NS, Boyd KV, Coopersmith CM (2008) Extracorporeal membrane oxygenation: current clinical practice, coding, and reimbursement. Chest 134:179–184
Pesenti A, Zanella A, Patroniti N (2009) Extracorporeal gas exchange. Curr Opin Crit Care 15:52–58
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363
Brogan TV, Thiagarajan RR, Rycus PT, Bartlett RH, Bratton SL (2009) Extracorporeal membrane oxygenation in adults with severe respiratory failure: a multi-center database. Intensive Care Med 35:2105–2114
Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler R (2009) Critically ill patients with 2009 influenza A (H1N1) infection in Canada. JAMA 302:1872–1879
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB (2008) Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358:1327–1335
Vassilakopoulos T, Zakynthinos S, Roussos C (2006) Bench-to-bedside review: weaning failure—should we rest the respiratory muscles with controlled mechanical ventilation? Crit Care 10:204
Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP (2009) Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 373:1874–1882
Sinderby C, Navalesi P, Beck J, Skrobik Y, Comtois N, Friberg S, Gottfried SB, Lindstrom L (1999) Neural control of mechanical ventilation in respiratory failure. Nat Med 5:1433–1436
Sinderby C (2003) Ventilatory assist driven by patient demand. Am J Respir Crit Care Med 168:729–730
Lellouche F, Brochard L (2009) Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, SmartCare). Best Pract Res Clin Anaesthesiol 23:81–93
Beck J, Reilly M, Grasselli G, Mirabella L, Slutsky AS, Dunn MS, Sinderby C (2009) Patient-ventilator interaction during neurally adjusted ventilatory assist in very low birth weight infants. Pediatr Res 65:663–668
Barwing J, Ambold M, Linden N, Quintel M, Moerer O (2009) Evaluation of the catheter positioning for neurally adjusted ventilatory assist. Intensive Care Med 35:1809–1814
Colombo D, Cammarota G, Bergamaschi V, De Lucia M, Corte FD, Navalesi P (2008) Physiologic response to varying levels of pressure support and neurally adjusted ventilatory assist in patients with acute respiratory failure. Intensive Care Med 34:2010–2018
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Sinderby C, Beck J, Spahija J, de Marchie M, Lacroix J, Navalesi P, Slutsky AS (2007) Inspiratory muscle unloading by neurally adjusted ventilatory assist during maximal inspiratory efforts in healthy subjects. Chest 131:711–717
Sessler CN, Gosnell MS, Grap MJ, Brophy GM, O’Neal PV, Keane KA, Tesoro EP, Elswick RK (2002) The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 166:1338–1344
Peek GJ, Moore HM, Moore N, Sosnowski AW, Firmin RK (1997) Extracorporeal membrane oxygenation for adult respiratory failure. Chest 112:759–764
Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli R, Rossi F, Iapichino G, Romagnoli G, Uziel L, Agostoni A et al (1986) Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 256:881–886
Mols G, Loop T, Geiger K, Farthmann E, Benzing A (2000) Extracorporeal membrane oxygenation: a ten-year experience. Am J Surg 180:144–154
Putensen C, Hering R, Muders T, Wrigge H (2005) Assisted breathing is better in acute respiratory failure. Curr Opin Crit Care 11:63–68
Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, Mutz N (2001) Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med 164:43–49
Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, Fernandez R, de la Cal MA, Benito S, Tomas R et al (1995) A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. N Engl J Med 332:345–350
Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE (1977) Control of breathing using an extracorporeal membrane lung. Anesthesiology 46:138–141
Brander L, Sinderby C, Lecomte F, Leong-Poi H, Bell D, Beck J, Tsoporis JN, Vaschetto R, Schultz MJ, Parker TG, Villar J, Zhang H, Slutsky AS (2009) Neurally adjusted ventilatory assist decreases ventilator-induced lung injury and non-pulmonary organ dysfunction in rabbits with acute lung injury. Intensive Care Med 35:1979–1989
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karagiannidis, C., Lubnow, M., Philipp, A. et al. Autoregulation of ventilation with neurally adjusted ventilatory assist on extracorporeal lung support. Intensive Care Med 36, 2038–2044 (2010). https://doi.org/10.1007/s00134-010-1982-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-1982-6