Abstract
Objective
Intrapulmonary percussive ventilation (IPV) is a form of high-frequency ventilation that can be superimposed on spontaneous breathing or conventional ventilation. Drawbacks include difficulties achieving adequate airway humidification and an inability to monitor delivered volumes and pressures, which may vary with patient characteristics. The objectives of this study were to assess various humidification set-ups, to measure intrapulmonary pressures and volumes resulting from IPV superimposed on a conventional driving ventilator (DV) and to test several ventilators regarding their ability to accept added IPV.
Design
Bench study in a test-lung set-up was used to measure humidification and the effects of adding IPV to a DV under various conditions of compliance, resistance, plateau and positive end-expiratory pressures. Then, five ventilators were tested in combination with IPV.
Measurements and results
Adequate humidification required a heated humidifier on the inspiratory line downstream of the IPV device. IPV increased end-inspiratory intrapulmonary pressures up to 10 cmH2O, increased delivered volumes up to 237 ml and generated intrinsic PEEP from 1.7 to 4.3 cmH2O when no PEEP was set on the DV. Intrinsic PEEP was lower or absent when PEEP was set on the DV. With most tested ventilators, IPV prevented reliable flow monitoring. Autotriggering and missing cycles were common and the PEEP effect varied across DVs.
Conclusion
Achieving adequate humidification with IPV requires a specific set-up. Superimposing IPV on standard ventilation can increase intrapulmonary pressures and tidal volumes importantly and interfere with the triggering sensors of the ventilator. These factors must be taken into account before clinical use.
Similar content being viewed by others
Introduction
High-frequency percussive ventilation is a mode of high-frequency ventilation that delivers small bursts of gas at rates higher than 1 Hz (usually 4–10 Hz). Two approaches have been used for high-frequency percussive ventilation. One uses high-frequency ventilation to achieve adequate gas exchange, while potentially limiting ventilator-induced injury [1]. In cohort studies of patients with acute respiratory distress syndrome failing conventional mechanical ventilation or with smoke inhalation injury, high-frequency percussive ventilation improved the PaO2/FiO2 ratio [2, 3]. However, the use of high-frequency percussive ventilation has been limited by a number of disadvantages including the absence of the monitoring data usually available with conventional mechanical ventilation and the considerable complexity of the settings. Intrapulmonary percussive ventilation (IPV, Percussionnaire Corporation®, Sandpoint, ID) is another approach characterised by simpler settings, as only driving pressure and percussion frequency must be adjusted. Very small volumes dependent on the driving pressure are administered at a frequency that is controlled by the operator. IPV can be superimposed on spontaneous breathing, mainly for physiotherapy or superimposed on conventional ventilation. Several studies used IPV during spontaneous breathing in patients with chronic obstructive disease [4, 5] or diseases characterised by excessive bronchial secretions such as Duchenne muscular dystrophy [6] or cystic fibrosis [7] because percussions could loosen secretions and improve their removal. Recently, IPV was added to conventional ventilation to recruit atelectatic areas of the lung [8]. Potential benefits of this hybrid technique may include secretion clearance, improved oxygenation and intermittent use. Several important technical issues remain unsolved, however, which limit the clinical use of IPV combined either with conventional invasive or non-invasive ventilation. We designed a study aimed at generating data that might help to overcome the limitations of IPV. During high-frequency ventilation, the high inspiratory flow rate and gas decompression prevent optimal humidification and warming of the inspired gas, increasing the risk of airway obstruction. Given the potentially severe consequences of unrecognised under-humidification during mechanical ventilation [9], we first designed a bench study to assess the efficacy of various humidification devices when IPV was added to a conventional ventilator. Absolute and relative humidities were measured with the nebuliser supplied with the IPV device, a heat-and-moisture exchanger (HME), an active HME (AHME) and a heated humidifier placed at different locations on the inspiratory line. Secondly, we designed an extensive bench evaluation of intrapulmonary pressures and volumes resulting from the addition of IPV to conventional ventilation under various conditions of respiratory system resistance and compliance, and with various ventilatory parameters. Thirdly, we tested the behaviour of several currently available ICU ventilators in response to the addition of IPV. Results have been presented in part at the 20th annual congress of the ESICM [10, 11].
Methods
Equipment and settings
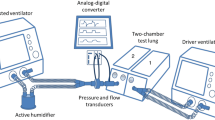
IPV is a form of high-frequency ventilation that delivers small volumes produced by a piston mechanism, the Phasitron®, which is driven by high-pressure gas at frequencies of 1–5 Hz. The Venturi effect drags humidified gas from a nebuliser through the Phasitron® to the patient. In our study, IPV was superimposed on a conventional ventilator, referred to as the driving ventilator (DV). When used in this way, the expiratory valve of the Phasitron® must be closed to prevent expired gas from draining through the Phasitron®. Gas bursts are delivered continuously throughout the respiratory cycles delivered by the DV. The driving gas is a mixture of air and oxygen. The driving pressure can be adjusted to obtain the desired magnitude of the gas bursts. Driving pressure and frequency are monitored on IPV. For our study, the inspiratory and expiratory lines of the DV and the IPV device were connected to a three-pronged connector, whose stem was connected to a test lung (see the electronic supplementary material for more details).
The DV used in the first two parts of the study (evaluation of humidification systems and measurements of pressures and volumes) was an 8,400 STI ventilator (Bird Corporation, Palm Springs, CA) with the following settings: square flow with a peak flow rate of 60 l/min, respiratory rate of 15 min−1, tidal volume (VT) of 500 ml and zero end-expiratory pressure. The ventilator had no tubing compliance compensation.
The IPV device was set to deliver high-frequency percussive ventilation at a rate of 5 Hz, which was the highest available frequency. The driving pressure was 1.2 bar and the inspiratory/expiratory ratio was fixed at 1/2.5 and not adjustable.
Humidification tests
The nebuliser recommended by the IPV device manufacturer for humidification was inserted in the circuit. We tested four humidification systems: a heated humidifier MR850 (Fisher & Paykel, Auckland, New Zealand) placed on the inspiratory line upstream of the IPV device (Fig. 1a), a heat-and-moisture exchanger (Hygrobac S, Covidien, Mansfield, MA) placed between the three-pronged connector and the test lung (Fig. 1b), an AHME (Hygrovent-Booster, Medisize, Hillegom, The Netherlands) placed between the three-pronged connector and the test lung (same set-up as in Fig. 1b) and a heated humidifier (MR 850) placed on the inspiratory line downstream of the IPV device (Fig. 1c). Because HMEs work by preserving the humidity from the patient’s expiration, the expired gas coming from the test lung in the experiments done with the HME and AHME was artificially humidified with a heated humidifier (MR 850) to mimic the natural humidity from the lungs [9]. Six set-ups were tested, namely, the heated humidifier upstream of the IPV device with and without the nebuliser, the HME and AHME without the nebuliser, the heated humidifier downstream of the IPV device without the nebuliser and the nebuliser alone.
Diagrams of the four humidification systems tested. DV Driving ventilator, HH heated humidifier, HME heat-and-moisture exchanger and AHME active heat-and-moisture exchanger, IPV intrapulmonary percussive ventilation. a (circuit 1) A heated humidifier was placed on the inspiratory line of the DV, upstream of the IPV device. b (circuits 2 and 3) An HME or active HME was positioned between the three-pronged connector and the test lung, downstream of the IPV device, and a heated humidifier in the test lung replicated the humidity and temperature conditions of a real lung. c (circuit 4) A heated humidifier was placed on the inspiratory line of the DV, downstream of the IPV device. The expiratory branch of the three-pronged connector was occluded
Humidity and temperature measurements
Mean ambient air temperature during the study period was 22.6 ± 0.2°C. All humidity measurements were performed using the psychrometric method after 3 h at steady-state [9, 12]. A device composed of two unidirectional valves to separate inspiratory and expiratory gas flows was inserted between the test lung and the humidifier. Two thermal probes, one dry and one wet, were placed in the inspiratory part of the device and monitored on a multi-channel high-precision (0.1°C) thermometer with digital reading (μR1000, Yokogawa electric corporation, Yamanashi-ken, Japan). For each condition, two measurements were obtained at the ventilator output and at the three-pronged connector on the inspiratory line. Temperatures at the heated humidifier inlet and outlet chambers and at the heated plate were carefully monitored. Ambient air temperature was measured using a high-precision (0.1°C) thermometer with digital reading (Duotemp, Fischer & Paykel, Auckland, New Zealand). Absolute and relative humidities were calculated for all the devices tested.
Bench study of pressures and volumes
Both the IPV and the DV generate volume and pressure, whose addition might modify intra-alveolar pressures during inspiration and expiration. We designed an extensive bench study to measure changes in DV-administered VTs and intra-alveolar pressures under various ventilatory and pulmonary conditions. Ventilatory parameters (pressures and volumes) were measured using the humidifier system that performed best in the first phase.
The circuit was connected to a single-compartment lung simulator (Michigan test lung, Michigan Instruments, Grand Rapids, MI) used with three compliance loads (C = 20, 50 and 100 ml/cmH2O) and three resistive loads (R = 5, 20 and 50 cmH2O/L/S Pneumoflo resistor, Michigan Instruments). Three inspiratory plateau durations (0, 0.2 and 0.4 s) were set on the DV and two IPV driving pressures (1.2 and 1.8 bar) were tested under each condition. As a result, nine compliance load/resistive load combinations were tested, each with six inspiratory plateau/driving pressure combinations.
To assess the effect of IPV when PEEP was set on the DV, the same parameters were recorded with different levels of PEEP (0, 5, 10 and 15 cmH2O) and 0 s inspiratory plateau. Resistive loads of 5 and 20 cmH2O/L/S were tested with compliances of 20 and 50 ml/cmH2O. Maximum alveolar pressure and VT due to IPV with different PEEP levels were measured from the recordings.
Pressure at the three-pronged connector was taken to represent the maximum airway opening pressure (Paomax) and the mean airway pressure (Paomean), pressure in the single-compartment test lung was taken to represent the maximum alveolar pressure (Palvmax) and the mean alveolar pressure (Palvmean). Mean pressure was measured over the whole respiratory cycle. Pressures were recorded using differential pressure transducers (Validyne, MP-45 ± 87 cmH2O, Northridge, CA). Flow was measured at the test-lung airway opening using a pneumotachograph connected to a differential pressure transducer (Sensym ICT, Milpitas, CA). The frequency response of this set-up was linear in the range of frequencies studied. Data were fed through a 12-bit analog-to-digital converter (Biopac MP 100 Goleta, CA), sampled at 200 Hz, and stored in a personal computer for further analysis.
Data collection
Under all the study conditions, the DV was started first, and after five steady-state respiratory cycles the IPV device was turned on for subsequent recordings. At least five cycles were recorded with the IPV device running under stable conditions. Palvmax, Palvmean, Paomax and Paomean were measured and VT was computed by integration of the flow signal during inspiration. During expiration, positive pressure inside the test lung (intrinsic PEEP) and peak-to-peak Pao were measured.
Study of ventilators
Several currently available ventilators were used as the DV to test their behaviour after addition of IPV. Three ventilators used wall pressure (Engström, General Electric, Madison, WI; Avea, Viasys Healthcare, Palm Springs, CA; and Evita 4, Dräger, Lubeck, Germany) and two used a turbine (Elisée 350 Res Med, Savigny Le Temple, France; and T-Bird, Viasys Healthcare, Palm Springs, CA). The ventilators were used in volume-controlled mode with square flow, a peak flow rate at 60 l/min, a respiratory rate of 15 min−1, and a VT of 500 ml. The combination of 20 cmH2O/L/S resistance with 40 ml/cmH2O compliance was tested with four PEEP levels (0, 5, 10 and 15 cmH2O).
Statistical analysis
Absolute and relative humidities under the various conditions were compared using the nonparametric Kruskal–Wallis test. P values less than 0.05 were considered significant. Results are given as median and interquartile. All pressures and volumes shown are mean of five consecutive cycles (Fig. 2).
Recordings of flow, pressure at the three-pronged connector (Pao) and pressure inside the test lung (Palv) during five cycles, including two cycles with the driving ventilator alone and three with the driving ventilator and the IPV device. VT tidal volume measured by integration of flow, Pao max maximal pressure at the three-pronged connector taken to represent maximal pressure at airway opening, Pao mean mean pressure at the Y-piece connector during a respiratory cycle, Palv max maximal pressure measured at the end of inspiration on the test lung side port taken to represent maximum alveolar pressure, Palv mean mean alveolar pressure inside the test lung during a respiratory cycle, PEEPalv positive end-expiratory pressure measured inside the test lung. All measurements were performed with and without the IPV device running to allow determination of the additional pressures and volumes generated with the IPV device
Results
Humidity
Absolute humidity of fully saturated air at 37°C is 44 mgH2O/l. This value would be achieved by the ideal humidifier. Humidity values with each of the four systems tested are reported in Table 1. In terms of airway humidity, the nebuliser provided with the IPV device was the least effective system when used alone. The only system that provided adequate absolute humidity was the heated humidifier placed on the inspiratory line downstream of the IPV device (Fig. 1c).
Ventilator parameters
Volume and pressure data with an IPV driving pressure of 1.2 bar are reported in Tables 2, 3 and 4. IPV increased the VT administered from a minimum of 7 ml to a maximum of 237 ml, increased Palvmax from 3 to 10 cmH2O, Palvmean from 2.7 to 5.3 cmH2O, Paomax from 5 to 8 cmH2O and Paomean from 2.4 to 4.9 cmH2O, with all resistance and compliance conditions and with all ventilator settings. The additional Palvmax due to the IPV device was smaller when compliance or resistance was increased. Increasing the plateau duration increased the additional VT due to IPV, for instance from 56 to 155 ml with a resistive load of 5 cmH2O/L/S and a compliance of 20 ml/cmH2O.
Increasing the IPV driving pressure to 1.8 bar increased the high-frequency burst volumes and then increased all volumes and pressures. IPV increased the VT delivered by the DV from a minimum of 39 ml to a maximum of 402 ml, increased Palvmax from 4 to 12 cmH2O, and increased Paomax from 12 to 15 cmH2O. Details are available in the electronic supplementary material.
During expiration, IPV generated intrinsic PEEP (from a minimum of 1.7 cmH2O to a maximum of 4.3 cmH2O). This intrinsic PEEP increased with the resistive load and with compliance, and was also higher with 1.8 bar than with 1.2 bar of driving pressure.
When external PEEP (higher than 5 cmH2O) was set on the DV, no additional intrinsic PEEP was generated by adding the IPV device. Palvmax showed smaller increases with than without PEEP (Fig. 3). Table 4 reports the effect of the various parameters on pressure and volume.
Measure of maximal alveolar pressure (Palvmax) with various levels of external PEEP set on the driving ventilator with and without the IPV device running. Maximal alveolar pressure (Palvmax) measured inside the test lung with (squares) and without (triangles) the IPV device running and with external PEEP set on the driving ventilator at 0, 5, 10 or 15 cmH2O. Palvmax increased with increasing PEEP but the influence of IPV was stable. Resulting PEEP (circles) is the total PEEP measured with IPV running. Using this driving ventilator, when PEEP is set on the DV, the resulting PEEP is not the sum of PEEP set + PEEP generated by IPV
Study of ventilators
Each of the tested ventilators exhibited a specific pattern of behaviour in response to IPV in terms of triggering, cycling-off and PEEP. The flow added to the circuit by the IPV device made spirometry unreliable.
-
With the Avea ventilator, PEEP higher than 10 cmH2O was associated with continuous autotriggering that required setting the flow trigger above 3 l/min. IPV did not generate intrinsic PEEP when the DV was set at 5 cmH2O or more.
-
With the Elisée ventilator, IPV had no influence on triggering and generated no intrinsic PEEP when the DV was set at 5 cmH2O or more.
-
With the Evita 4, dysregulation of the respiratory rate occurred, with prolonged cycles (7 s instead of 4 s set on the DV) and occasional PEEP drops during IPV with all PEEP levels set on the DV. When PEEP was set on the DV, the resulting intrinsic PEEP inside the test lung was added to the PEEP due to the IPV device.
-
With the Engström ventilator, continuous autotriggering occurred when PEEP was set at 10 cmH2O. Changing the trigger sensitivity did not change autotriggering at this level of PEEP. Autotriggering did not occur when PEEP was set at 5 cmH2O. No additional intrinsic PEEP was noted when the DV was set at 5 cmH2O or more.
-
Finally, with the T-Bird ventilator, no autotriggering was noted. When PEEP was set on the DV, the resulting intrinsic PEEP inside the test lung was added to the PEEP generated by the IPV device.
Discussion
The main results of this bench study can be summarised as follows: (1) adding IPV to conventional ventilation markedly reduces humidity and providing adequate humidity requires placement of a heated humidifier on the inspiratory line downstream of the IPV device, (2) the IPV device behaves as an additional ventilator that adds pressures and volumes to tidal ventilation, (3) IPV generates intrinsic PEEP, whose level depends on the DV used and extrinsic PEEP level and (4) recently developed ventilators often show abnormal function when used in combination with IPV.
Humidity
A major concern in intubated patients receiving high-frequency ventilation is provision of adequate humidification and warming of the inspired gases [13]. With high-velocity gases, the Venturi effect decreases temperature and relative humidity [14, 15]. Adding IPV to conventional ventilation decreases the humidity of the delivered gases. Acceleration of a large amount of ambient gas results in a drop in gas temperature, even larger with the IPV-device nebulizer (system 1). Adequate absolute humidity was achieved with only one of the four set-ups tested in our study, namely, a heated humidifier placed on the inspiratory line downstream of the IPV device (Fig. 1c). The minimal humidity level recommended during prolonged mechanical ventilation is 30 mgH2O/l [16]. Because absolute humidity was low with conventional circuits in which the IPV device was placed just downstream of the three-pronged connector, we tested a system in which a heated humidifier was placed downstream of the IPV device to avoid cooling due to gas mixing and acceleration. This set-up allowed to achieve an adequate level of hygrometry, which is necessary to facilitate secretion removal [17]. Heating and humidifying the IPV driving gases (using Vapotherm® for example) have not been tested because the Phasitron® is expected to accelerate the gases and decrease temperature and absolute humidity. Using a longer inspiratory line between the IPV device and the three-pronged connector may attenuate the percussion wave shape.
Ventilator parameters
Flow measurements were recorded using a screen pneumotachograph that provided linear values within the frequency range studied. Flow and pressure values were recorded at three-pronged connector (representing the airway opening) and inside the test lung (representing the alveoli). In a recent bench study of high-frequency percussive ventilation, pressures and volume were influenced by resistive load, whereas elastic load affected only VT [18]. Because of the complexity of ventilator settings in this study (which involved high- and low-frequency, inspiratory/expiratory ratios, oscillatory continuous positive airway pressure and driving pressure), the results are difficult to apply to clinical situation. Therefore, we studied IPV added to a DV that required only two adjustments, i.e. driving pressure and percussion frequency. In keeping with the previous study [18], volume and pressure were heavily dependent on resistive load, whereas compliance influenced volumes more than pressures. Furthermore, we confirmed that IPV generated intrinsic PEEP, and we quantified the pressure and volume increases due to the addition of IPV.
The IPV device behaves like an additional ventilator that adds volumes during the respiratory cycles. Pressures and volumes measured inside the test lung were increased as a result of the added continuous (percussive) flow. Insufflation duration during conventional ventilation directly influenced the amount of flow added in the circuit, as shown by the increase in VT as plateau duration increased. IPV generated 2–3 cmH2O of PEEP, in keeping with results obtained by superimposing IPV on spontaneous breathing [5]. Unfortunately, PEEP generated by IPV and PEEP set on the DV cannot be simply added, as total PEEP depends on the PEEP-regulating algorithm of the DV. IPV can deeply affect both cycling and PEEP regulation of new-generation ventilators. Furthermore, flow monitoring must be inactivated, preventing calculation of inspired and expired volumes. Paomean, which can be potentially monitored on ventilators, underestimates the increases in Palv mean secondary to IPV (Table 3). Therefore, each DV must be subjected to extensive bench testing designed to evaluate its compatibility with IPV (especially cycling-off and trigger) and the level of PEEP when IPV is used concomitantly. In addition, this system may be used from adults to neonates. Even if the relevance of small extravolume delivered by IPV may be limited regarding the adult population, it may be injurious for a premature baby.
We are aware of a single clinical study of IPV added to a DV [8]. Obese patients with acute respiratory distress syndrome or acute lung injury and compression atelectasis not improved by conventional ventilation received IPV added to pressure-controlled ventilation. PaO2/FiO2 and atelectasis as assessed by computed tomography improved with IPV. Pure oxygen was used as the driving gas for IPV. As shown in our study, IPV adds volume, so that the FiO2 provided to the patient depends on both the IPV driving gas and the FiO2 set on the DV. Moreover, part of the PaO2/FiO2 increase observed in the clinical study [8] may be ascribable to increases in VT and pressures.
Conclusion
Adding IPV to conventional ventilation requires a specific circuit to ensure that humidification is adequate. Administered pressures and volumes increase depending on patient resistance and compliance. Knowledge of respiratory mechanics is needed to estimate the pressures and volumes actually administered to the patient, especially when compliance is low, as in acute respiratory distress syndrome.
References
Hurst JM, Branson RD, Davis K Jr, Barrette RR, Adams KS (1990) Comparison of conventional mechanical ventilation and high-frequency ventilation. A prospective, randomized trial in patients with respiratory failure. Ann Surg 211:486–491
Reper P, Wibaux O, Van Laeke P, Vandeenen D, Duinslaeger L, Vanderkelen A (2002) High frequency percussive ventilation and conventional ventilation after smoke inhalation: a randomised study. Burns 28:503–508
Velmahos GC, Chan LS, Tatevossian R, Cornwell EE 3rd, Dougherty WR, Escudero J, Demetriades D (1999) High-frequency percussive ventilation improves oxygenation in patients with ARDS. Chest 116:440–446
Vargas F, Bui HN, Boyer A, Salmi LR, Gbikpi-Benissan G, Guenard H, Gruson D, Hilbert G (2005) Intrapulmonary percussive ventilation in acute exacerbations of COPD patients with mild respiratory acidosis: a randomized controlled trial [ISRCTN17802078]. Crit Care 9:R382–389
Nava S, Barbarito N, Piaggi G, De Mattia E, Cirio S (2006) Physiological response to intrapulmonary percussive ventilation in stable COPD patients. Respir Med 100:1526–1533
Toussaint M, De Win H, Steens M, Soudon P (2003) Effect of intrapulmonary percussive ventilation on mucus clearance in duchenne muscular dystrophy patients: a preliminary report. Respir Care 48:940–947
Varekojis SM, Douce FH, Flucke RL, Filbrun DA, Tice JS, McCoy KS, Castile RG (2003) A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high-frequency chest wall compression in hospitalized cystic fibrosis patients. Respir Care 48:24–28
Tsuruta R, Kasaoka S, Okabayashi K, Maekawa T (2006) Efficacy and safety of intrapulmonary percussive ventilation superimposed on conventional ventilation in obese patients with compression atelectasis. J Crit Care 21:328–332
Lellouche F, Taille S, Maggiore SM, Qader S, L’Her E, Deye N, Brochard L (2004) Influence of ambient and ventilator output temperatures on performance of heated-wire humidifiers. Am J Respir Crit Care Med 170:1073–1079
Dellamonica J, Lyazidi A, Vargas F, Brochard L (2007) Humidification during intrapulmonary percussive ventilation (IPV) added to a conventional ventilator. Intensive Care Med 33(Suppl 2):S136
Dellamonica J, Louis B, Vargas F, Brochard L (2007) Bench testing of intrapulmonary percussive ventilation added to a conventional ventilator: pressures and volumes generated. Intensive Care Med 33(Suppl 2):S137
Lacherade JC, Auburtin M, Cerf C, Van de Louw A, Soufir L, Rebufat Y, Rezaiguia S, Ricard JD, Lellouche F, Brun-Buisson C, Brochard L (2005) Impact of humidification systems on ventilator-associated pneumonia: a randomized multicenter trial. Am J Respir Crit Care Med 172:1276–1282
Kahn RC (1983) Humidification of the airways. Adequate for function and integrity? Chest 84:510–511
Chalon J, Loew DA, Malebranche J (1972) Effects of dry anesthetic gases on tracheobronchial ciliated epithelium. Anesthesiology 37:338–343
Shelly MP, Lloyd GM, Park GR (1988) A review of the mechanisms and methods of humidification of inspired gases. Intensive Care Med 14:1–9
AARC clinical practice guideline (1992) Humidification during mechanical ventilation. American Association for Respiratory Care. Respir Care 37:887–890
Chatburn RL (2007) High-frequency assisted airway clearance. Respir Care 52:1224–1235 (Discussion 1235–1237)
Lucangelo U, Antonaglia V, Zin WA, Fontanesi L, Peratoner A, Bird FM, Gullo A (2004) Effects of mechanical load on flow, volume and pressure delivered by high-frequency percussive ventilation. Respir Physiol Neurobiol 142:81–91
Acknowledgment
We thank “International Medical Assistance by Percussionaire”, which provided the machine for the bench tests.“International Medical Assistance by Percussionaire” reimbursed the registration fees to a scientific meeting for one of us.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dellamonica, J., Louis, B., Lyazidi, A. et al. Intrapulmonary percussive ventilation superimposed on conventional ventilation: bench study of humidity and ventilator behaviour. Intensive Care Med 34, 2035–2043 (2008). https://doi.org/10.1007/s00134-008-1190-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-008-1190-9