Abstract
Objective
Reactive oxygen species have been implicated in the pathogenesis of hypoxia–reoxygenation injury. However, little information is known regarding the temporal profile of cerebral hydrogen peroxide (HPO) production and its response to N-acetylcysteine (an antioxidant) administration during neonatal hypoxia–reoxygenation. Using an acute swine model of neonatal hypoxia–reoxygenation, we examined the short-term neuroprotective effects of N-acetylcysteine on cerebral HPO production and oxidative stress in the brain.
Design
Controlled, block-randomized animal study.
Setting
University animal research laboratory.
Subjects
Newborn piglets (1–3 days, 1.7–2.1 kg).
Interventions
At 5 min after reoxygenation, piglets were given either saline or N-acetylcysteine (20 or 100 mg/kg/h) in a blinded, randomized fashion.
Measurements and results
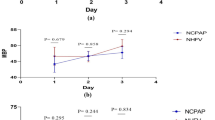
Newborn piglets were block-randomized into a sham-operated group (without hypoxia–reoxygenation, n = 5) and three hypoxic–reoxygenated groups (2 h of normocapnic alveolar hypoxia followed by 2 h of reoxygenation, n = 7/group). Heart rate, mean arterial pressure, cortical HPO concentration, amino acid levels in cerebral microdialysate, and cerebral tissue glutathione and lipid hydroperoxide levels were examined. Hypoxic piglets were hypotensive and acidotic, and they recovered similarly in all hypoxic–reoxygenated groups. In hypoxic–reoxygenated control piglets, the cortical HPO concentration gradually increased during reoxygenation. Both doses of N-acetylcysteine abolished the increased HPO concentration and oxidized glutathione levels and tended to reduce the glutathione ratio and lipid hydroperoxide levels in the cerebral cortex (p = 0.08 and p = 0.1 vs. controls, respectively). N-acetylcysteine at 100 mg/kg/h also increased the cerebral extracellular taurine levels.
Conclusion
In newborn piglets with hypoxia–reoxygenation, postresuscitation administration of N-acetylcysteine reduces cerebral HPO production and oxidative stress, probably through a taurine-related mechanism.




Similar content being viewed by others
References
Richter-Landsberg C, Vollgraf U (1998) Mode of cell injury and death after hydrogen peroxide exposure in cultured oligodendroglia cells. Exp Cell Res 244:218–229
Whittemore ER, Loo DT, Watt JA, Cotman CW (1995) A detailed analysis of hydrogen peroxide-induced cell death in primary neuronal culture. Neuroscience 67:921–932
Lafemina MJ, Sheldon RA, Ferriero ADM (2006) Acute hypoxia–ischemia results in hydrogen peroxide accumulation in neonatal but not adult mouse brain. Pediatr Res 59:680–683
Fullerton HJ, Ditelberg JS, Chen SF, Sarco DP, Chan PH, Epstein CJ, Ferriero DM (1998) Copper/zinc superoxide dismutase transgenic brain accumulates hydrogen peroxide after perinatal hypoxia ischemia. Ann Neurol 44:357–364
Lei B, Adachi N, Arai T (1997) The effect of hypothermia on H2O2 production during ischemia and reperfusion: a microdialysis study in the gerbil hippocampus. Neurosci Lett 222:91–94
Patt A, Harken AH, Burton LK, Rodell TC, Piermattei D, Schorr WJ, Parker NB, Berger EM, Horesh IR, Terada LS (1988) Xanthine oxidase-derived hydrogen peroxide contributes to ischemia reperfusion-induced edema in gerbil brains. J Clin Invest 81:1556–1562
Banaclocha MM (2001) Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypo 56:472–477
Zafarullah M, Li WQ, Sylvester J, Ahmad M (2003) Molecular mechanisms of N-acetylcysteine actions. Cell Mol Life Sci 60:6–20
Khan M, Sekhon B, Jatana M, Giri S, Gilg AG, Sekhon C, Singh I, Singh AK (2004) Administration of N-acetylcysteine after focal cerebral ischemia protects brain and reduces inflammation in rat model of experimental stroke. J Neurosci Res 76:519–527
Wang X, Svedin P, Nie C, Lapatto R, Zhu C, Gustavsson M, Sandberg M, Karlsson JO, Romero R, Hagberg H, Mallard C (2007) N-acetylcysteine reduces lipopolysaccharide-sensitized hypoxic-ischemic brain injury. Ann Neurol 61:263–271
Lee TF, Jantzie LL, Todd KG, Cheung P-Y (2007) Postresuscitation administration of N-acetylcysteine attenuates cerebral free radical generation during reoxygenation of hypoxic newborn piglets. E-PAS:618443.8 (abstract)
Ortalani O, Conti A, De Gaudio AR, Moraldi E, Cantini Q, Novelli G (2000) The effect of glutathione and N-acetylcysteine on lipoperoxidative damage in patients with early septic shock. Am J Respir Crit Care Med 161:1907–1911
Haase E, Bigam DL, Nakonechny QB, Jewell LD, Korbutt G, Cheung PY (2004) Resuscitation with 100% oxygen causes intestinal glutathione oxidation and reoxygenation injury in asphyxiated newborn piglets. Ann Surg 240:364–373
Martin LJ, Brambrink A, Koehler RC, Traystman RJ (1997) Primary sensory and forebrain motor systems in the newborn brain are preferentially damaged by hypoxia–ischemia. J Comp Neurol 377:262–285
Richards JG, Todd KG, Emara M, Haase E, Cooper SL, Bigam DL, Cheung PY (2006) A dose-response study of graded reoxygenation on the carotid haemodynamics, matrix metalloproteinase-2 activities and amino acid concentrations in the brain of asphyxiated newborn piglets. Resuscitation 69:319–327
Johnson ST, Bigam DL, Emara M, Obaid L, Slack G, Korbutt G, Korbutt G, Jewell LD, Van Aerde J, Cheung PY (2007) N-acetylcysteine improves the hemodynamics and oxidative stress in hypoxic newborn pigs reoxygenated with 100% oxygen. Shock 2007 Jun 17 [Epub ahead of print]
Jantzie LL, Rauw GA, Todd KG (2006) The effects of doxycycline administration on amino acid neurotransmitters in an animal model of neonatal hypoxia–ischemia. Neurochem Inter 49:771–728
Parent M, Bush D, Rauw G, Master S, Vaccarino F, Baker G (2001) Analysis of amino acids and catecholamines, 5-hydroxytryptamine and their metabolites in brain areas in the rat using in vivo microdialysis. Methods 23:11–20
Hyslop PA, Zhang Z, Pearson DV, Phebus LA (1995) Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with cytotoxic potential of H2O2 in vitro. Brain Res 671:181–186
Lei B, Adachi N, Arai T (1998) Measurement of the extracellular H2O2 in the brain by microdialysis. Brain Res Protoc 3:33–36
Kutzsche S, Ilves P, Kirkeby OJ, Saugstad OD (2001) Hydrogen peroxide production in leukocytes during cerebral hypoxia and reoxygenation with 100% or 21% oxygen in newborn piglets. Pediatr Res 49:834–842
Sprong RC, Winkelhuyzen-Janssen AM, Aarsman CJ, van Oirschot JF, van der Bruggen T, van Asbeck BS (1998) Low dose N-acetylcysteine protects rats against endotoxin-mediated oxidative stress, but high-dose increase mortality. Am J Respir Crit Care Med 157:1283–1293
Szkudlarek U, Zdziechowski A, Witkowski K, Kasielski M, Luczynska M, Luczynski R, Sarniak A, Nowak D (2004) Effect of inhaled N-acetylcysteine on hydrogen peroxide exhalation in healthy subjects. Pulm Pharmacol Ther 17:155–162
Palmer C, Menzies SL, Roberts RL, Pavlick G, Connor JR (1999) Changes in iron histochemistry after hypoxic-ischemic brain injury in neonatal rat. J Neurosci Res 56:60–71
Caglikulekci M, Dirlik M, Pata C, Plasse M, Tamer L, Ogetman Z, Ercan B (2006) Effect of N-acetylcysteine on blood and tissue lipid peroxidation in lipopolysaccharide-induced obstructive jaundice. J Invest Surg 19:175–184
Kingston R, Kelly CJ, Murray P (2004) The therapeutic role of taurine in ischemia–reperfusion injury. Curr Pharm Design 10:2401–2410
Estevez AY, O'Regan MH, Song D, Phillis JW (1999) Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem Res 24:447–452
Andine P, Sandberg M, Bagenholm R, Lehmann A, Hagberg H (1991) Intra- and extracellular changes of amino acids in the cerebral cortex of the neonatal rat during hypoxic-ischemia. Brain Res Dev Brain Res 64:115–120
Huppi PS, Posse S, Lazeyras F, Burri R, Bossi E, Herschkowitz N (1991) Magnetic resonance in preterm and term newborns: 1H-spectroscopy in developing human brain. Pediatr Res 30:574–578
Wharton BA, Morley R, Isaacs EB, Cole TJ, Lucas A (2004) Low plasma taurine and later neurodevelopment. Arch Dis Child Fetal Neonatal Ed 89:F497–498
Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, Esteras A, Llombart-Bosch A, Morcillo EJ (2001) Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J 17:1228–1235
Muhling J, Nickolaus KA, Halabi M, Fuchs M, Krull M, Engel J, Wolff M, Matejec R, Langefeld TW, Welters ID, Menges T, Dehne MG, Sablotzki A, Hempelmann G (2005) Alterations in neutrophil (PMN) free intracellular alpha-keto a profiles and immune functions induced by L-alanyl-L-glutamine, arginine or taurine. Amino Acids 29:289–300
Sekhon B, Sehkon C, Khan M, Patel SJ, Singh I, Singh AK (2003) N-acetyl cysteine protects against injury in a rat model of focal cerebral ischemia. Brain Res 971:1–8
Zhang QG, Tian H, Li HC, Zhang GY (2006) Antioxidant N-acetylcysteine inhibits the activation of JNK3 mediated by the GluR6-PSD95-MLK3 signaling module during cerebral ischemia in rat hippocampus. Neurosci Lett 408:159–164
Acknowledgements
This project was funded by an operating grant from the Canadian Institutes of Health Research (MOP-CSB-53009) and a grant-in-aid from the George and Dorothy Davey Endowment for Brain Injury Research. P.Y.C. is an investigator with the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research. L.L.J. is a recipient of a Canada Graduate Scholarship from the Natural Science and Engineering Research Council of Canada and a doctoral research award from the Alberta Heritage Foundation of Medical Research. There is no financial conflict of interest in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
We are very grateful to Corinne Cote for her excellent assistance and technical expertise.
Rights and permissions
About this article
Cite this article
Lee, TF., Jantzie, L.L., Todd, K.G. et al. Postresuscitation N-acetylcysteine treatment reduces cerebral hydrogen peroxide in the hypoxic piglet brain. Intensive Care Med 34, 190–197 (2008). https://doi.org/10.1007/s00134-007-0880-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0880-z