Abstract
Background
Inhaled nitric oxide (iNO) has been used for treatment of acute respiratory failure and pulmonary hypertension since 1991 in adult patients in the perioperative setting and in critical care.
Methods
This contribution assesses evidence for the use of iNO in this population as presented to a expert group jointly organised by the European Society of Intensive Care Medicine and the European Association of Cardiothoracic Anaesthesiologists.
Conclusions
Expert recommendations on the use of iNO in adults were agreed on following presentation of the evidence at the expert meeting held in June 2004.
Similar content being viewed by others
Introduction
Inhaled nitric oxide (iNO) has been used in Europe for treatment of acute respiratory failure and pulmonary hypertension for several years, both in the operating room and the intensive care unit. In the middle 1980s Higenbotham and his group [1, 2] were the first to demonstrate that iNO selectively decreases pulmonary artery pressure (PAP) in a series of patients with primary pulmonary hypertension. Then it was demonstrated that iNO can selectively reverse experimental pulmonary arterial hypertension [3]. In the early 1990s it was shown that iNO selectively decreases pulmonary arterial hypertension and improves arterial oxygenation in patients with acute respiratory distress syndrome (ARDS) [4, 5, 6, 7, 8, 9]. The rationale for the treatment of critically ill patients with iNO was based on these studies [1, 2, 3, 4, 5, 6, 7, 8, 9]. However, subsequent randomised controlled trials (RCTs) failed to confirm an improvement in survival or morbidity in critically ill patients treated with iNO [10, 11, 12, 13, 14, 15]. In addition, to date there is no drug approval for these indications in adults, although iNO is still extensively used as an off-label drug, and many clinicians consider it an important treatment, combining effective selective pulmonary vasodilatation with a favourable pharmacological profile [16]. iNO has been approved for treatment of term and near-term neonates with hypoxic respiratory failure. The provision of a pharmaceutical product has led to high drug costs and an increased need for justification of the clinical use of iNO in adults in daily practice. This suggested to our group that recommendations should be established on the use of iNO in adults covering all aspects of current and potential applications based on expert opinion.
Methods
An Advisory Board was established under the auspices of the European Society of Intensive Care Medicine and European Association of Cardiothoracic Anaesthesiologists to coordinate the scientific program of the meeting. The board consisted of experts with proven scientific or clinical expertise relevant to the clinical use of iNO. The board identified a further panel of experts who were invited to act as section leaders whose role was to review the literature in their designated subject area, taking special care to ensure the presence of different opinions. Section leaders were asked to produce written summaries of their subject area, which were then circulated to delegates prior to the meeting and which formed the basis of the evidence presented to delegates at the expert meeting itself. A further panel of opinion leaders were invited to attend the meeting on the basis of their known interest in the use of iNO or their status as opinion leaders in the field of adult intensive care. The European Society of Intensive Care Medicine and the European Society of Cardiothoracic Anaesthesiologists were officially represented at the meeting. At the expert meeting each subject area was presented in summary by the section leader(s), following which open discussions led to the composition of draft expert recommendations statements. These were then edited and re-presented to delegates with further discussion and reading leading to final agreement on the individual recommendations.
The first part of this program was built upon discussions among a core group of experts, and this led to draft recommendations covering areas such as clinical pharmacology, toxicity, dosing, administration and various indications supported by appropriate literature and clinical data analysis. These draft recommendations were made available to a wider group of physicians through a dedicated restricted website. Following discussions based upon these statements revisions were published online. A 2-day conference was then organised, enabling 58 experts from different specialties and coming from 14 European Union countries to openly discuss all related issues and jointly agree on recommendations. During this conference an Editorial Committee was formed to summarise expert recommendations. These statements were then published anew on the dedicated, restricted website for final review and comments by all participants. Following a last round of online discussions the Editorial Committee prepared the final article which is presented in this contribution. The cost of this project, including hotel and accommodation, travel, online conferencing facilities, IT support and website, expenses for preparation work, was approx. €218,000 (€20,000 for the first part and €198,000 for the second part of the program) which was supported through an unrestricted grant from INO Therapeutics. The process of producing the present expert recommendations was entirely independent of the sponsoring company, and the contributors specified their potential conflicts of interest. The sponsor has no authorship or editorial control over the content of the meetings or any subsequent publication. Most of the expense for this effort has been time by the Committee.
Results
Clinical pharmacology
iNO acutely relaxes constricted vascular smooth muscle leading to vasodilatation of the pulmonary circulation with no measurable haemodynamic action outside the lung (‘selective pulmonary vasodilatation’). In addition, iNO potentially dilates constricted bronchial smooth muscle, and it may improve arterial oxygenation in hypoxaemic patients by reducing the intrapulmonary shunt leading to enhanced matching of ventilation and perfusion. The selective pulmonary vasodilator action of iNO has been confirmed in various animal models [3], in a human model of acute alveolar hypoxia [17] and in patients with pulmonary arterial hypertension resulting from pulmonary vascular constriction [1, 2, 18, 19]. However, due to its short half-life sustained vasodilatation requires the continuous delivery of iNO to the lungs. Sudden disruption of iNO therapy can therefore result in a severe withdrawal reaction with rebound and possibly severe vasoconstriction [20]. The bronchodilator effect of iNO is dose dependent in anaesthetised animals [21] and in volunteers or patients with active bronchoconstriction [22]. Even at the high doses of iNO used in these studies (80 ppm) the bronchodilator response of iNO was less effective than a subsequent inhalation of a standard β2-agonist [22].
Several clinical studies have tested the use of iNO for treatment of acute pulmonary hypertension or hypoxaemia employing doses between 3 and 80 ppm. However, these acute physiological effects did not alter clinical outcome parameters, such as mortality or morbidity, and a high proportion of patients do not respond to iNO therapy (non-responders). There is some evidence from experimental and human studies for potential pharmacodynamic effects outside the pulmonary circulation, mainly on diuresis and natriuresis [23, 24], platelet function [25, 26], and modulation of the immune response [27].
Expert recommendations:
-
It has been conclusively demonstrated in human experimentation and clinical studies that exposure to iNO causes a concentration-dependent and immediate selective pulmonary vasodilatation in the presence of pulmonary vasoconstriction in most patients.
-
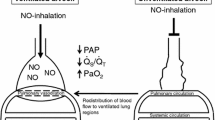
Nitric oxide induces vasorelaxation in ventilated portions of the lung and redistributes pulmonary blood flow, thus reducing intrapulmonary shunting in most hypoxaemic patients, at concentrations ranging from 0.1–10 ppm iNO. However, the optimum dose may vary over time and between different subjects.
-
iNO is believed to have other pulmonary and extrapulmonary effects. Their clinical relevance and concentration-response relationships remain to be investigated.
Synergistic effects
The rationale for combining iNO with other therapeutics, either pharmacological or nonpharmacological, is to obtain a synergistic or additive effect on pulmonary vascular tone in patients with pulmonary arterial hypertension or hypoxaemia. Most proposed synergistic drugs are effective in influencing only one or the other of these two potential therapeutic aims. For example, prostacyclin [28] and adenosine [29] directly stimulate the synthesis of cyclic adenosine monophosphate (cAMP) whereas phosphodiesterases inhibitors inhibit the breakdown of cAMP and cyclic guanosine monophosphate (cGMP), thereby effecting pulmonary vascular relaxation through signalling pathways that are different from those which are directly brought about by NO.
Expert recommendations:
-
The rationale for combining iNO with other drugs is to obtain an additive (or synergistic) effect and to induce an additional reduction in pulmonary vascular tone and/or further optimisation of pulmonary gas exchange than is obtained by use of iNO alone.
-
There are clinical reports of the co-administration of ‘synergistic’ drugs with iNO. The majority of synergistic drugs are effective in influencing only one or another of these desired therapeutic aims [30, 31, 32, 33, 34, 35, 36, 37].
-
Only the association of iNO and inhaled nebulised prostacyclin has shown, in a limited number of patients, positive effects on both pulmonary hypertension and gas exchange [36]. The therapeutic benefit of this synergistic response has yet to be determined.
-
Published studies on the use of potentially synergistic drugs in association with iNO report effects on small populations or are methodologically inadequate.
-
Dose-response studies with both iNO and the associated drugs are incompletely defined.
-
The underlying molecular mechanisms of interaction between NO and potentially synergistic drugs and the cross-talk pathways of the two drugs acting together are only partially understood.
-
The interpretation of clinical data in individual patients and from small published experiences must therefore be made with caution.
-
On the basis of current evidence the clinical use of synergistic drugs in adults in association with iNO cannot be recommended outside the confines of clinical trials.
Toxicology, monitoring, delivery, transport
Over the course of the past decade iNO has been administered to numerous patients without any apparent major side effect [10, 11, 12, 13, 14, 15]. Although the use of iNO is considered to be safe, and there is no evidence of direct NO toxicity at clinically relevant doses, precautions and safety regulations must be taken into account, especially the risk of exposure to higher oxides of NO (i.e. nitric dioxide). Therefore care should be taken to use iNO in humans that has been manufactured according to agreed good manufacturing practice standards (medical grade iNO). These issues have been reviewed in detail previously, including toxicology, monitoring of iNO therapy, delivery and procedures for transport of patients on iNO together with environmental issues and considerations on staff training [38].
Toxicology and monitoring
Expert recommendations:
-
There is no evidence of direct NO toxicity at clinically relevant doses.
-
Methaemoglobin should be measured 4 h after commencing iNO and daily thereafter.
-
Clinically significant levels of methaemoglobin are unlikely to result unless iNO concentrations over 20 ppm are administered.
-
Administration of iNO is associated with NO2 formation which is potentially toxic.
-
Environmental exposure limits exposure to NO2 to a 2-ppm 8 h time-weighted average in non-intubated patients and staff.
-
Clinically significant levels of NO2 are unlikely to occur when iNO is delivered by an efficient delivery system at concentrations of 20 ppm or less.
-
If long-term iNO treatment is to be undertaken, attempts should be made to reduce the concentration of iNO to 10 ppm or less to further reduce exposure to potentially toxic NO2.
-
Use of iNO is associated with accumulation of nitrate and nitrite. The significance of these increases is uncertain.
-
There are no long-term follow-up studies from which freedom from late adverse effects following iNO therapy can be ascertained.
Delivery
Expert recommendations:
-
INO should be delivered by a system approved for clinical use, conforming to appropriate CE standards and capable of meeting the following specifications.
-
It should be able to deliver a constant concentration of iNO to the patient.
-
The design should minimise the generation of NO2 and should have continuous monitoring and alarms for inspired NO, NO2 and O2.
-
A backup system for hand ventilation should be immediately available to ensure continuous iNO delivery in the case of delivery device malfunction.
-
The delivery device should be compatible with the type(s) of ventilator(s) in use, which at present does not include closed-circuit, rebreathing systems during anaesthesia.
Transport
Expert recommendations:
-
iNO therapy must be delivered without interruption when patients are transferred within or between hospitals.
-
An iNO delivery and monitoring system which is of low weight and is designed and approved for use during transport in road and air ambulances is urgently needed.
Contraindication
-
Methaemoglobin reductase deficiency (congenital or acquired)
Diagnostic assessment
Heart failure
The frequently elevated PVR in patients with chronic left ventricular failure may be a result of dysregulation of vascular smooth muscle tone and structural remodelling [39]. There is growing evidence that the dysregulation of pulmonary vascular tone in disease states, such as chronic heart failure involves vascular endothelial dysfunction with impaired endogenous NO availability in the pulmonary circulation. Endothelial cell dysfunction predisposes the vessel wall to vasoconstriction, leucocyte adherence, platelet activation, mitogenesis, thrombosis, impaired coagulation and vascular inflammation [40]. In addition, endothelial function testing may serve as a useful biomarker of pulmonary circulatory function [41]. Bocchi and colleagues [42] reported sudden development of pulmonary oedema in patients with severe congestive heart failure treated with iNO, which was most probably due to a sudden increase in left atrial filling caused by pulmonary vasodilatation rather than a direct negative inotropic effect of iNO [43]. iNO may be used as a test for pulmonary vasoreactivity before cardiac transplantation.
Expert recommendations:
-
Response to iNO treatment may identify patients still suitable for heart or heart/lung transplantation or to help to identify patients with congenital heart disease suitable for further intervention.
-
iNO decreases PVR but potentially increases left ventricular preload which may be dangerous in left ventricular dysfunction. In the presence of left heart dysfunction it is increasingly recognised that iNO testing should be performed only after optimising heart failure therapy immediately prior to testing.
-
iNO testing is useful to demonstrate the remaining reactivity of the precapillary component of postcapillary pulmonary hypertension. Reduction in PAP/PVR shown by iNO testing does not imply that long-term iNO therapy should be instituted
Pulmonary arterial hypertension
Pulmonary arterial hypertension, previously known as primary pulmonary hypertension, is a rapidly progressive disease of the pulmonary vasculature with consecutive right heart failure [44]. Prognosis may be improved in adult patients responding to calcium channel blocker and/or anticoagulation [45] and in patients treated with continuous prostacyclin [46]. The response to acute vasodilator testing has important implications both for the choice of therapy and for prognosis [28, 47, 48]. For example, only patients with a positive response to acute vasodilator testing remain suitable for long-term treatment with calcium channel blocker. Those who do not are treated with long-term intravenous epoprostenol. Today intravenous epoprostenol adenosine or iNO is recommended for acute vasodilator testing in adults, defined as a decrease in the mean PAP of at least 10 mmHg to less than 40 mmHg with an increased or unchanged cardiac output [49]. iNO has been shown to be superior to prostacyclin for this use [50] whereas aerosolised iloprost is more effective in improving oxygenation and haemodynamics in patients with primary pulmonary hypertension [51]. Combining oxygen and iNO can identify a greater number of appropriate candidates for corrective cardiac surgery or transplantation during preoperative testing [52].
Expert recommendations:
-
iNO is a potent selective pulmonary vasodilator which used alone or in combination with other vasodilators may be useful in revealing the extent of reversibility (if any) in selected patients with pulmonary arterial hypertension.
-
iNO clearly identifies responders suitable for long-term treatment with calcium channel blockers.
-
iNO dose recommended for acute vasodilator testing should be 10–20 ppm. iNO does not have relevant adverse effects during short-term acute testing.
-
iNO combined with additional O2 may lead to further pulmonary vasodilatation.
-
There is insufficient data to recommend iNO for long-term therapy of pulmonary arterial hypertension.
Medical conditions complicated by pulmonary arterial hypertension
Thromboembolism
iNO, which decreases PAP [2], is likely to unload the right ventricle in pulmonary embolism and chronic thromboembolic pulmonary hypertension. Furthermore, its platelet anti-aggregate property could prove beneficial [53, 54]. iNO for severe pulmonary embolism or chronic thromboembolic pulmonary hypertension has not been investigated in randomised controlled trials. Animal studies have shown that iNO decreases PVR [55] and platelet aggregation [54]. Use of iNO has been reported in case reports from patients with massive pulmonary embolism leading to cardiogenic shock. iNO decreased right ventricular afterload, improved cardiac output (CO) and increased arterial oxygen content [56, 57, 58]. After thrombendarterectomy iNO significantly improved arterial oxygenation but had a negligible effect on PAP. In one case postoperative hypotension progressively reversed with iNO [59].
Expert recommendations:
-
There are no controlled trials which support the routine use of iNO in patients with thromboembolic disease.
-
iNO might be of benefit in selected patients with thromboembolic disease who have severe right ventricular failure and/or severe hypoxaemia.
Sickle-cell disease
Stiffened red blood cells lead to impaired blood flow in the microcirculation, veno-occlusive phenomena, inflammation and haemolysis [60]. Given the depletion of endogenous NO by cell-free haemoglobin iNO may restore endothelial homeostasis by enhancing pulmonary vasodilatation and inactivation of cell-free haemoglobin. In transgenic sickle-cell mice iNO protects from hypoxia/reoxygenation induced lung injury, attenuates inflammatory response, modulates genes involved in ischaemic/reperfusion injury and improves survival [61]. Case reports in the acute chest syndrome have shown an improved oxygenation and decreased PAPm with iNO [62, 63]. A small prospective, double-blind, randomised, placebo-controlled paediatric study has shown that iNO is associated with a greater reduction in pain and less use of morphine over the first 6 h but not duration of hospitalisation [64].
Expert recommendations:
-
There is limited clinical experience suggesting that iNO improves oxygenation and decreases PVR in some patients with acute chest syndrome.
-
A single randomised, placebo-controlled trial in severe vaso-occlusive disease suggests that use of iNO is associated with improved pain control.
-
At present there are insufficient data to recommend the routine use of iNO to manage complications of sickle cell disease.
Chronic obstructive pulmonary disease
In chronic obstructive pulmonary disease pulmonary hypertension responds poorly to oxygen therapy and has an adverse impact on prognosis. Pathophysiological studies in stable patients have consistently documented that iNO lowers PAPm and PVR [65]. Studies report both an improvement [65] and a worsening in arterial oxygenation [66]. The concomitant administration of O2 either prevents a decrease in [67] or increases PaO2 [33]. In intubated and mechanically ventilated patients neither arterial oxygenation nor cardiac function are influenced by iNO [68]. A recent prospective randomised trial comparing O2 alone and O2 plus iNO over a 3-month period in stable patients demonstrated a reduction in PAPm and PVR and an increase in cardiac output at the end of the trial period in the O2 plus iNO group only [69]. No study to date has explored the impact of either acute or chronic iNO on patient outcome.
Expert recommendations:
-
There is no evidence that iNO therapy is of clinical benefit in patients with chronic obstructive pulmonary disease.
Cardiac surgery
Perioperative pulmonary hypertension in adult cardiac surgery
Several strategies have been employed to avoid postoperative pulmonary hypertension, including pharmacological inhibitors of inflammation, improved cardioplegic solutions, and new surgical techniques avoiding the use of CPB. Treatment strategies include the use of iNO, prostaglandins and ultimately ventricular assist devices.
CPB has been shown to reduce NO production within the pulmonary vasculature, and replacement of endogenous NO by treatment with iNO lowers the increased vascular resistance [70] and reduces markers of CPB-induced inflammatory activation [71]. iNO was more effective than milrinone in lowering PVR in 45 adult cardiac surgery patients [72]. iNO is equally effective as standard intravenous vasodilators, without altering systemic haemodynamics [73]. Doses greater than 20 ppm offer no advantage [73, 74], and patients with a high preoperative PVR have a greater response to iNO [75]. Similar data have been reported for treatment of pulmonary hypertension in high-risk cardiac surgery patients [19], patients undergoing heart transplantation [76, 77] and insertion of left ventricular assist devices [78, 79].
Expert recommendations:
-
There are no randomised, placebo-controlled clinical trials that show that iNO improves clinical outcomes in adults with perioperative acute right ventricular dysfunction and elevated PVR
-
Clinical experience suggests that in patients with confirmed acute right ventricular dysfunction and elevated PVR, use of iNO may result in haemodynamic improvement when used during or after cardiac surgery
-
Prior to iNO administration right ventricular function should be optimised with conventional treatment (specifically, ensuring optimal ventilation, thoracic decompression, preload optimisation, attempting to lower PVR with standard measures, increasing systemic perfusion pressure to increase coronary perfusion, and reduction in myocardial oxygen consumption).
Left ventricular assist devices
Ventricular assist devices (VAD) can dramatically improve survival and morbidity of patients with severe acute or chronic heart failure, either as a bridge to transplantation, as a bridge to recovery or as a permanent therapy [80]. In the presence of pulmonary hypertension filling of the left-sided VAD is impaired and the right ventricle cannot be unloaded further worsening the right ventricular dysfunction. This situation may necessitate the implant of a biventricular assist device. The development of right ventricular dysfunction remains a serious clinical problem being associated with a high transfusion rate, multiple organ failure, increased length of stay and a high mortality rate [80]. Recent studies ranging from case reports, observational studies and RCTs have demonstrated beneficial effects of iNO therapy in such patients [43, 81, 82]. However, there are no data to suggest that this transient action of iNO has any lasting effect that favourably influences clinical outcomes. The doses used in theses studies varied between 10–40 ppm. Potential concerns arise from the possibility of abrupt rebound pulmonary hypertension from withdrawal of iNO, and sudden increases in left atrial filling, similar to the mechanisms occurring in patients with acute heart failure [43]. Currently the role of iNO in the treatment of heart failure patients undergoing left-sided VAD insertion is tested in an ongoing large, multicentre trial.
Expert recommendations:
-
There is a high prevalence of right ventricular dysfunction refractory to conventional clinical measures in patients in whom insertion of a left ventricular assist device is required
-
The expert panel believe that iNO therapy is effective in providing favourable pulmonary haemodynamics leading to improved right ventricular and left-sided VAD assisted cardiac output in patients with pulmonary hypertension and inadequate left-sided VAD flow refractory to conventional manoeuvres. On the basis of these improved critical physiological variables the expert panel recommend that it is reasonable to consider the use of iNO in this clinical situation among other vasodilator therapies.
-
Further studies are required to better define the indications for iNO in cardiac surgical patients and in particular to elucidate its effects on clinical outcomes.
Heart transplantation
Cardiac transplantation remains the surgical option for treatment of end-stage heart disease. In many cases severe pulmonary hypertension is present at time of transplantation, contributing to life-threatening right heart failure, a significant predictor of early postoperative mortality [83]. iNO reduced elevated pulmonary resistance in adult cardiac transplantation [84], was more effective in reducing PVR than intravenous prostaglandin E1 [85], reduced the occurrence of right ventricular failure [76] and increased survival irrespective of the preoperative condition in a large case series of patients undergoing cardiac transplantation [77]. Until now there are no randomised clinical trials determining the role of iNO for the treatment of pulmonary hypertension in cardiac transplant patients. However, iNO therapy is considered to be a part of a multimodal treatment strategy by expert opinion, including iNO, optimising right ventricular filling, increasing heart rate and reducing PVR [76].
Expert recommendations:
-
Several institutions with extensive experience in cardiac transplantation use and recommend iNO as a part of standard therapy for all cardiac transplant procedures associated with increased PVR.
-
Following weaning from the ventilator iNO therapy may be discontinued and be replaced by intravenous vasodilators.
Thoracic surgery
One-lung ventilation
Hypoxaemia frequently occurs during one-lung ventilation (OLV) and is due mainly to an increased pulmonary blood flow to the non-ventilated lung (intrapulmonary shunt) [86, 87]. High inspired oxygen concentrations, intermittent two-lung ventilation, continuous positive airway pressure and high frequency jet ventilation to the ventilated, dependent lung are common interventions to improve arterial oxygenation. Two studies suggest that in the absence of arterial hypoxaemia or pulmonary hypertension iNO does not modify oxygenation or pulmonary artery pressure [88, 89]. In theory iNO should increase pulmonary blood flow to the ventilated, dependent lung by dilating the pulmonary vasculature [90]. However, Fradj et al. [91] reported that iNO at 20 ppm was not superior to nitrogen in the treatment of arterial hypoxaemia during OLV. In another study iNO administration was effective only in a subgroup of patients with severe hypoxaemia, high intrapulmonary shunt and pulmonary hypertension during OLV [92].
Almitrine combined with iNO can attenuate arterial hypoxaemia during OLV or in patients with ARDS [30, 31, 34, 93, 94, 95]. However, as iNO during OLV is seldom effective when used alone, the role of iNO to improve the effects of almitrine has yet to be confirmed [94]. Therefore the expert panel cannot make any recommendations on the use of a combination of iNO with intravenous almitrine, taking also into consideration the long half-life time of the drug, its potential for systemic toxicity and its limited availability in most countries.
Expert recommendations:
-
There is no evidence to support the routine use of iNO for the prevention or reversal of hypoxaemia during OLV.
-
Some patients who develop very severe hypoxaemia during OLV which is refractory to conventional management may benefit from iNO.
Ischaemia-reperfusion injury
Postpneumonectomy pulmonary oedema is a potential complication of lung resection with an associated mortality of 50–100%. Age, side of lung resection, volume of fluids infused perioperatively, preoperative lung function, use of fresh-frozen plasma, mechanical ventilatory support and the extent of mediastinal lymphatic dissection all contribute to the risk of developing postpneumonectomy pulmonary oedema. During OLV relative ischaemia of the non-ventilated lung is followed by re-expansion and reperfusion of the remaining lung tissue leading to ischaemia-reperfusion with oxidative damage and lung injury [96]. iNO has been shown to be of therapeutic value in patients with postpneumonectomy lung injury by selectively dilating the pulmonary vasculature and improving ventilation/perfusion mismatch and oxygenation [97, 98]. However, the role of iNO in preventing ischaemia-reperfusion injury during clinical lung transplantation remains controversial [99]. Two uncontrolled clinical studies suggest that NO prevents ischaemia-reperfusion injury [77, 100]. These findings were not confirmed by a randomised, double-blinded, placebo-controlled trial studying the effect of iNO (20 ppm) starting 10 min after reperfusion on mortality and physiological variables in patients undergoing lung transplantation [101].
Expert recommendations:
-
There is no evidence that iNO administered during reperfusion prevents or attenuates the development of ischaemia/reperfusion injury after lung transplantation or thrombendarterectomy.
-
It remains to be determined whether iNO applied alone or together with anti-inflammatory agents administered prior to or at reperfusion modulates reperfusion injury in humans.
-
There is evidence from one RCT that iNO may reduce the need of cardiopulmonary bypass in sequential bilateral lung transplantation by improving haemodynamic stability or oxygenation during clamping of the recipients pulmonary artery or following reperfusion of the firstly implanted lung [102].
ARDS/ALI
In acute lung injury (ALI) and ARDS there is a marked maldistribution of pulmonary perfusion in favour of poorly or non-ventilated lung areas. iNO therapy offers the possibility to selectively modulate the pulmonary blood flow, reduce pulmonary hypertension and improve matching of ventilation to perfusion [4]. Early clinical trials of iNO demonstrated improved arterial oxygenation and pulmonary haemodynamics in patients with ARDS [4, 5, 6, 7, 8, 9, 103]. Further clinical studies demonstrated that combining iNO with positive end-expiratory pressure or prone positioning augments the beneficial effects on arterial oxygenation [33, 104, 105], thus pointing to a valuable role for iNO in combination with recruitment manoeuvres for treatment of severe hypoxaemia in patients with ARDS.
Subsequent RCTs involving over 900 patients with ALI/ARDS confirmed a significant increase in arterial oxygenation in the majority of patients without any sign of clinically relevant side effects. However, this increase in PaO2 was only transient, and no improvement in important outcome parameters such as mortality and ventilator-free days was found [10, 11, 12, 13, 15, 106].
With respect to the available data the expert panel felt it reasonable to use iNO as a rescue treatment in patients with severe refractory hypoxaemia. This interpretation is essentially suggested by a recent meta-analysis of RCTs on iNO therapy [107]. Aerosolised prostaglandins have been shown to exert similar effects as iNO therapy in improving arterial oxygenation and/or pulmonary haemodynamics and may offer an alternative form of therapy [108, 109].
Expert recommendations:
-
iNO improves arterial oxygenation and haemodynamics in most ARDS patients in the acute phase. However, there is no evidence of any beneficial effect of iNO beyond the first 24–72 h of therapy, and no benefit on clinical outcomes has been demonstrated by at least four published randomised trials of ARDS.
-
However, RCTs provide no evidence that iNO affects mortality in ALI/ARDS, and its routine use in these conditions cannot be recommended
-
Available RCTs do not resolve the question of whether iNO leads to any clinically significant benefits (such as improved survival, increased ventilator-free days and reduced use of extracorporeal membrane oxygenation) in certain subgroups of patients, such as those with severe hypoxaemia not responding to conventional treatment.
-
The expert group considered that, based on the known physiological effects of iNO in ARDS and whilst awaiting evidence from further clinical trials, it is reasonable to use iNO as a rescue treatment in patients with severe refractory hypoxaemia
Conclusion
These recommendations were compiled by an interdisciplinary panel of European experts in the field of iNO therapy. They are designed to promote the safe use of this therapy and to suggest areas in which iNO therapy may be of benefit in adults. In summary, the expert panel identified a variety of medical conditions in which the use of iNO as a rescue treatment in patients with severe acute pulmonary arterial hypertension and/or severe refractory arterial hypoxaemia is reasonable. In addition iNO is a useful drug for testing pulmonary vasoreactivity in patients undergoing heart transplantation or in patients with pulmonary arterial hypertension. It is hoped that these recommendations will encourage evidence-based practice and further clinical trials on the use of iNO therapy in adults.
References
Higenbottam T, Pepke-Zaba J, Scott J, Woolman P, Coutts C, Wallwork J (1988) Inhaled endothelial derived-relaxing factor (EDRF) in primary pulmonary hypertension (PPH). Am Rev Respir Dis [Suppl]:137:A107
Pepke-Zaba J, Higenbottam TW, Dinh-Xuan AT, Stone D, Wallwork J (1991) Inhaled nitric oxide as a cause of selective pulmonary vasodilatation in pulmonary hypertension. Lancet 338:1173–1174
Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM (1991) Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation 83:2038–2047
Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM (1993) Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med 328:399–405
Abman SH, Griebel JL, Parker DK, Schmidt JM, Swanton D, Kinsella JP (1994) Acute effects of inhaled nitric oxide in children with severe hypoxemic respiratory failure. J Pediatr 124:881–888
Bigatello LM, Hurford WE, Kacmarek RM, Roberts JD Jr, Zapol WM (1994) Prolonged inhalation of low concentrations of nitric oxide in patients with severe adult respiratory distress syndrome. Effects on pulmonary hemodynamics and oxygenation. Anesthesiology 80:761–770
Gerlach H, Pappert D, Lewandowski K, Rossaint R, Falke KJ (1993) Long-term inhalation with evaluated low doses of nitric oxide for selective improvement of oxygenation in patients with adult respiratory distress syndrome. Intensive Care Med 19:443–449
Puybasset L, Stewart T, Rouby JJ, Cluzel P, Mourgeon E, Belin MF, Arthaud M, Landault C, Viars P (1994) Inhaled nitric oxide reverses the increase in pulmonary vascular resistance induced by permissive hypercapnia in patients with acute respiratory distress syndrome. Anesthesiology 80:1254–1267
Young JD, Brampton WJ, Knighton JD, Finfer SR (1994) Inhaled nitric oxide in acute respiratory failure in adults. Br J Anaesth 73:499–502
Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C (1999) Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med 25:911–919
Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, Elstad MR, Campbell EJ, Troyer BE, Whatley RE, Liou TG, Samuelson WM, Carveth HJ, Hinson DM, Morris SE, Davis BL, Day RW (1998) Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med 157:1372–1380
Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, Francoeur M, Charbonneau M, Blaise G (1998) Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med 157:1483–1488
Dellinger RP, Zimmerman JL, Taylor RW, Straube RC, Hauser DL, Criner GJ, Davis K Jr, Hyers TM, Papadakos P (1998) Effects of inhaled nitric oxide in patients with acute respiratory distress syndrome: results of a randomized phase II trial. Inhaled Nitric Oxide in ARDS Study Group. Crit Care Med 26:15–23
Gerlach H, Rossaint R, Pappert D, Falke KJ (1993) Time-course and dose-response of nitric oxide inhalation for systemic oxygenation and pulmonary hypertension in patients with adult respiratory distress syndrome. Eur J Clin Invest 23:499–502
Taylor RW, Zimmerman JL, Dellinger RP, Straube RC, Criner GJ, Davis K Jr, Kelly KM, Smith TC, Small RJ (2004) Low-dose inhaled nitric oxide in patients with acute lung injury: a randomized controlled trial. JAMA 291:1603–1609
Beloucif S, Payen D (1998) A European survey of the use of inhaled nitric oxide in the ICU. Working Group on Inhaled NO in the ICU of the European Society of Intensive Care Medicine. Intensive Care Med 24:864–877
Frostell CG, Blomqvist H, Hedenstierna G, Lundberg J, Zapol WM (1993) Inhaled nitric oxide selectively reverses human hypoxic pulmonary vasoconstriction without causing systemic vasodilation. Anesthesiology 78:427–435
Roberts JD Jr, Lang P, Bigatello LM, Vlahakes GJ, Zapol WM (1993) Inhaled nitric oxide in congenital heart disease. Circulation 87:447–453
Girard C, Lehot JJ, Pannetier JC, Filley S, Ffrench P, Estanove S (1992) Inhaled nitric oxide after mitral valve replacement in patients with chronic pulmonary artery hypertension. Anesthesiology 77:880–883
Miller OI, Tang SF, Keech A, Celermajer DS (1995) Rebound pulmonary hypertension on withdrawal from inhaled nitric oxide. Lancet 346:51–52
Dupuy PM, Shore SA, Drazen JM, Frostell C, Hill WA, Zapol WM (1992) Bronchodilator action of inhaled nitric oxide in guinea pigs. J Clin Invest 90:421–428
Hogman M, Frostell CG, Hedenstrom H, Hedenstierna G (1993) Inhalation of nitric oxide modulates adult human bronchial tone. Am Rev Respir Dis 148:1474–1478
Wraight WM, Young JD (2001) Renal effects of inhaled nitric oxide in humans. Br J Anaesth 86:267–269
Wennmalm A, Benthin G, Edlund A, Jungersten L, Kieler-Jensen N, Lundin S, Westfelt UN, Petersson AS, Waagstein F (1993) Metabolism and excretion of nitric oxide in humans. An experimental and clinical study. Circ Res 73:1121–1127
Samama CM, Diaby M, Fellahi JL, Mdhafar A, Eyraud D, Arock M, Guillosson JJ, Coriat P, Rouby JJ (1995) Inhibition of platelet aggregation by inhaled nitric oxide in patients with acute respiratory distress syndrome. Anesthesiology 83:56–65
Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, Walsh-Sukys MC, McCaffrey MJ, Cornfield DN, Bhutani VK, Cutter GR, Baier M, Abman SH (1999) Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet 354:1061–1065
Wan S, LeClerc JL, Vincent JL (1997) Inflammatory response to cardiopulmonary bypass: mechanisms involved and possible therapeutic strategies. Chest 112:676–692
Olschewski H, Simonneau G, Galie N, Higenbottam T, Naeije R, Rubin LJ, Nikkho S, Speich R, Hoeper MM, Behr J, Winkler J, Sitbon O, Popov W, Ghofrani HA, Manes A, Kiely DG, Ewert R, Meyer A, Corris PA, Delcroix M, Gomez-Sanchez M, Siedentop H, Seeger W (2002) Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 347:322–329
Ralevic V, Burnstock G (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492
Jolliet P, Bulpa P, Ritz M, Ricou B, Lopez J, Chevrolet JC (1997) Additive beneficial effects of the prone position, nitric oxide, and almitrine bismesylate on gas exchange and oxygen transport in acute respiratory distress syndrome. Crit Care Med 25:786–794
Lu Q, Mourgeon E, Law-Koune JD, Roche S, Vezinet C, Abdennour L, Vicaut E, Puybasset L, Diaby M, Coriat P et al (1995) Dose-response curves of inhaled nitric oxide with and without intravenous almitrine in nitric oxide-responding patients with acute respiratory distress syndrome. Anesthesiology 83:929–943
Gillart T, Bazin JE, Cosserant B, Guelon D, Aigouy L, Mansoor O, Schoeffler P (1998) Combined nitric oxide inhalation, prone positioning and almitrine infusion improve oxygenation in severe ARDS. Can J Anaesth 45:402–409
Germann P, Poschl G, Leitner C, Urak G, Ullrich R, Faryniak B, Roder G, Kaider A, Sladen R (1998) Additive effect of nitric oxide inhalation on the oxygenation benefit of the prone position in the adult respiratory distress syndrome. Anesthesiology 89:1401–1406
Gallart L, Lu Q, Puybasset L, Umamaheswara Rao GS, Coriat P, Rouby JJ (1998) Intravenous almitrine combined with inhaled nitric oxide for acute respiratory distress syndrome. The NO Almitrine Study Group. Am J Respir Crit Care Med 158:1770–1777
Ziegler JW, Ivy DD, Wiggins JW, Kinsella JP, Clarke WR, Abman SH (1998) Effects of dipyridamole and inhaled nitric oxide in pediatric patients with pulmonary hypertension. Am J Respir Crit Care Med 158:1388–1395
Kuhlen R, Walbert E, Frankel P, Thaden S, Behrendt W, Rossaint R (1999) Combination of inhaled nitric oxide and intravenous prostacyclin for successful treatment of severe pulmonary hypertension in a patient with acute respiratory distress syndrome. Intensive Care Med 25:752–754
Rialp G, Betbese AJ, Perez-Marquez M, Mancebo J (2001) Short-term effects of inhaled nitric oxide and prone position in pulmonary and extrapulmonary acute respiratory distress syndrome. Am J Respir Crit Care Med 164:243–249
Macrae DJ, Field D, Mercier JC, Moller J, Stiris T, Biban P, Cornick P, Goldman A, Gothberg S, Gustafsson LE, Hammer J, Lonnqvist PA, Sanchez-Luna M, Sedin G, Subhedar N (2004) Inhaled nitric oxide therapy in neonates and children: reaching a European consensus. Intensive Care Med 30:372–380
Moraes DL, Colucci WS, Givertz MM (2000) Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 102:1718–1723
Verma S, Anderson TJ (2002) Fundamentals of endothelial function for the clinical cardiologist. Circulation 105:546–549
Celermajer DS, Cullen S, Deanfield JE (1993) Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation 87:440–446
Bocchi EA, Bacal F, Auler Junior JO, Carmone MJ, Bellotti G, Pileggi F (1994) Inhaled nitric oxide leading to pulmonary edema in stable severe heart failure. Am J Cardiol 74:70–72
Hare JM, Shernan SK, Body SC, Graydon E, Colucci WS, Couper GS (1997) Influence of inhaled nitric oxide on systemic flow and ventricular filling pressure in patients receiving mechanical circulatory assistance. Circulation 95:2250–2253
Galie N, Seeger W, Naeije R, Simonneau G, Rubin LJ (2004) Comparative analysis of clinical trials and evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol 43:81S-88S
Rich S, Kaufmann E, Levy PS (1992) The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med 327:76–81
Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH et al (1996) A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 334:296–302
Simonneau G, Barst RJ, Galie N, Naeije R, Rich S, Bourge RC, Keogh A, Oudiz R, Frost A, Blackburn SD, Crow JW, Rubin LJ (2002) Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med 165:800–804
Hoeper MM, Schwarze M, Ehlerding S, Adler-Schuermeyer A, Spiekerkoetter E, Niedermeyer J, Hamm M, Fabel H (2000) Long-term treatment of primary pulmonary hypertension with aerosolized iloprost, a prostacyclin analogue. N Engl J Med 342:1866–1870
Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A (2004) Clinical classification of pulmonary hypertension. J Am Coll Cardiol 43:5S–12S
Morales-Blanhir J, Santos S, de Jover L, Sala E, Pare C, Roca J, Rodriguez-Roisin R, Barbera JA (2004) Clinical value of vasodilator test with inhaled nitric oxide for predicting long-term response to oral vasodilators in pulmonary hypertension. Respir Med 98:225–234
Leuchte HH, Schwaiblmair M, Baumgartner RA, Neurohr CF, Kolbe T, Behr J (2004) Hemodynamic response to sildenafil, nitric oxide, and iloprost in primary pulmonary hypertension. Chest 125:580–586
Sablotzki A, Hentschel T, Gruenig E, Schubert S, Friedrich I, Muhling J, Dehne MG, Czeslick E (2002) Hemodynamic effects of inhaled aerosolized iloprost and inhaled nitric oxide in heart transplant candidates with elevated pulmonary vascular resistance. Eur J Cardiothorac Surg 22:746–752
Loscalzo J (2001) Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res 88:756–762
Kermarrec N, Zunic P, Beloucif S, Benessiano J, Drouet L, Payen D (1998) Impact of inhaled nitric oxide on platelet aggregation and fibrinolysis in rats with endotoxic lung injury. Role of cyclic guanosine 5′-monophosphate. Am J Respir Crit Care Med 158:833–839
Bottiger BW, Motsch J, Dorsam J, Mieck U, Gries A, Weimann J, Martin E (1996) Inhaled nitric oxide selectively decreases pulmonary artery pressure and pulmonary vascular resistance following acute massive pulmonary microembolism in piglets. Chest 110:1041–1047
Estagnasie P, Le Bourdelles G, Mier L, Coste F, Dreyfuss D (1994) Use of inhaled nitric oxide to reverse flow through a patent foramen ovale during pulmonary embolism. Ann Intern Med 120:757–759
Capellier G, Jacques T, Balvay P, Blasco G, Belle E, Barale F (1997) Inhaled nitric oxide in patients with pulmonary embolism. Intensive Care Med 23:1089–1092
Schenk P, Mittermayer C, Ratheiser K (1999) Inhaled nitric oxide in a patient with severe pulmonary embolism. Ann Emerg Med 33:710–714
Pinelli G, Mertes PM, Carteaux JP, Hubert T, Dopff C, Burtin P, Villemot JP (1996) Inhaled nitric oxide as an adjunct to pulmonary thromboendarterectomy. Ann Thorac Surg 61:227–229
Bunn HF (1997) Pathogenesis and treatment of sickle cell disease. N Engl J Med 337:762–769
Martinez-Ruiz R, Montero-Huerta P, Hromi J, Head CA (2001) Inhaled nitric oxide improves survival rates during hypoxia in a sickle cell (SAD) mouse model. Anesthesiology 94:1113–1118
Atz AM, Wessel DL (1997) Inhaled nitric oxide in sickle cell disease with acute chest syndrome. Anesthesiology 87:988–990
Sullivan KJ, Goodwin SR, Evangelist J, Moore RD, Mehta P (1999) Nitric oxide successfully used to treat acute chest syndrome of sickle cell disease in a young adolescent. Crit Care Med 27:2563–2568
Weiner DL, Hibberd PL, Betit P, Cooper AB, Botelho CA, Brugnara C (2003) Preliminary assessment of inhaled nitric oxide for acute vaso-occlusive crisis in pediatric patients with sickle cell disease. JAMA 289:1136–1142
Adnot S, Kouyoumdjian C, Defouilloy C, Andrivet P, Sediame S, Herigault R, Fratacci MD (1993) Hemodynamic and gas exchange responses to infusion of acetylcholine and inhalation of nitric oxide in patients with chronic obstructive lung disease and pulmonary hypertension. Am Rev Respir Dis 148:310–316
Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R (1996) Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet 347:436–440
Ashutosh K, Phadke K, Jackson JF, Steele D (2000) Use of nitric oxide inhalation in chronic obstructive pulmonary disease. Thorax 55:109–113
Baigorri F, Joseph D, Artigas A, Blanch L (1999) Inhaled nitric oxide does not improve cardiac or pulmonary function in patients with an exacerbation of chronic obstructive pulmonary disease. Crit Care Med 27:2153–2158
Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, Germann P, Block LH (2003) Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax 58:289–293
Morita K, Ihnken K, Buckberg GD, Sherman MP, Ignarro LJ (1996) Pulmonary vasoconstriction due to impaired nitric oxide production after cardiopulmonary bypass. Ann Thorac Surg 61:1775–1780
Gianetti J, Del Sarto P, Bevilacqua S, Vassalle C, De Filippis R, Kacila M, Farneti PA, Clerico A, Glauber M, Biagini A (2004) Supplemental nitric oxide and its effect on myocardial injury and function in patients undergoing cardiac surgery with extracorporeal circulation. J Thorac Cardiovasc Surg 127:44–50
Solina A, Papp D, Ginsberg S, Krause T, Grubb W, Scholz P, Pena LL, Cody R (2000) A comparison of inhaled nitric oxide and milrinone for the treatment of pulmonary hypertension in adult cardiac surgery patients. J Cardiothorac Vasc Anesth 14:12–17
Schmid ER, Burki C, Engel MH, Schmidlin D, Tornic M, Seifert B (1999) Inhaled nitric oxide versus intravenous vasodilators in severe pulmonary hypertension after cardiac surgery. Anesth Analg 89:1108–1115
Fullerton DA, Jones SD, Jaggers J, Piedalue F, Grover FL, McIntyre RC Jr (1996) Effective control of pulmonary vascular resistance with inhaled nitric oxide after cardiac operation. J Thorac Cardiovasc Surg 111:753–762–
Solina AR, Ginsberg SH, Papp D, Pantin EJ, Denny J, Ghandivel I, Krause TJ (2002) Response to nitric oxide during adult cardiac surgery. J Invest Surg 15:5–14
Stobierska-Dzierzek B, Awad H, Michler RE (2001) The evolving management of acute right-sided heart failure in cardiac transplant recipients. J Am Coll Cardiol 38:923–931
Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, Moriguchi J, Hamilton MA, Kobashigawa J, Laks H (2001) Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation 72:638–641
Mavroidis D, Sun BC, Pae WE Jr (1999) Bridge to transplantation: the Penn State experience. Ann Thorac Surg 68:684–67
Oz MC, Argenziano M, Catanese KA, Gardocki MT, Goldstein DJ, Ashton RC, Gelijns AC, Rose EA, Levin HR (1997) Bridge experience with long-term implantable left ventricular assist devices. Are they an alternative to transplantation? Circulation 95:1844–1852
Kavarana MN, Pessin-Minsley MS, Urtecho J, Catanese KA, Flannery M, Oz MC, Naka Y (2002) Right ventricular dysfunction and organ failure in left ventricular assist device recipients: a continuing problem. Ann Thorac Surg 73:745–750
Macdonald PS, Keogh A, Mundy J, Rogers P, Nicholson A, Harrison G, Jansz P, Kaan AM, Spratt P (1998) Adjunctive use of inhaled nitric oxide during implantation of a left ventricular assist device. J Heart Lung Transplant 17:312–316
Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, Smerling AJ, Oz MC (1998) Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg 65:340–345
Costard-Jackle A, Hill I, Schroeder JS, Fowler MB (1991) The influence of preoperative patient characteristics on early and late survival following cardiac transplantation. Circulation 84:III329–III337
Kieler-Jensen N, Milocco I, Ricksten SE (1993) Pulmonary vasodilation after heart transplantation. A comparison among prostacyclin, sodium nitroprusside, and nitroglycerin on right ventricular function and pulmonary selectivity. J Heart Lung Transplant 12:179–184
Rajek A, Pernerstorfer T, Kastner J, Mares P, Grabenwoger M, Sessler DI, Grubhofer G, Hiesmayr M (2000) Inhaled nitric oxide reduces pulmonary vascular resistance more than prostaglandin E(1) during heart transplantation. Anesth Analg 90:523–530
Benumof JL (1985) One-lung ventilation and hypoxic pulmonary vasoconstriction: implications for anesthetic management. Anesth Analg 64:821–833
Ullrich R, Bloch KD, Ichinose F, Steudel W, Zapol WM (1999) Hypoxic pulmonary blood flow redistribution and arterial oxygenation in endotoxin-challenged NOS2-deficient mice. J Clin Invest 104:1421–1429
Rich GF, Lowson SM, Johns RA, Daugherty MO, Uncles DR (1994) Inhaled nitric oxide selectively decreases pulmonary vascular resistance without impairing oxygenation during one-lung ventilation in patients undergoing cardiac surgery. Anesthesiology 80:57–62
Wilson WC, Kapelanski DP, Benumof JL, Newhart 2nd JW, Johnson FW, Channick RN (1997) Inhaled nitric oxide (40 ppm) during one-lung ventilation, in the lateral decubitus position, does not decrease pulmonary vascular resistance or improve oxygenation in normal patients. J Cardiothorac Vasc Anesth 11:172–176
Sticher J, Scholz S, Boning O, Schermuly RT, Schumacher C, Walmrath D, Hempelmann G (2002) Small-dose nitric oxide improves oxygenation during one-lung ventilation: an experimental study. Anesth Analg 95:1557–1562
Fradj K, Samain E, Delefosse D, Farah E, Marty J (1999) Placebo-controlled study of inhaled nitric oxide to treat hypoxaemia during one-lung ventilation. Br J Anaesth 82:208–212
Rocca GD, Passariello M, Coccia C, Costa MG, Di Marco P, Venuta F, Rendina EA, Pietropaoli P (2001) Inhaled nitric oxide administration during one-lung ventilation in patients undergoing thoracic surgery. J Cardiothorac Vasc Anesth 15:218–223
Wysocki M, Delclaux C, Roupie E, Langeron O, Liu N, Herman B, Lemaire F, Brochard L (1994) Additive effect on gas exchange of inhaled nitric oxide and intravenous almitrine bismesylate in the adult respiratory distress syndrome. Intensive Care Med 20:254–259
Moutafis M, Liu N, Dalibon N, Kuhlman G, Ducros L, Castelain MH, Fischler M (1997) The effects of inhaled nitric oxide and its combination with intravenous almitrine on Pao2 during one-lung ventilation in patients undergoing thoracoscopic procedures. Anesth Analg 85:1130–1135
Payen DM, Gatecel C, Plaisance P (1993) Almitrine effect on nitric oxide inhalation in adult respiratory distress syndrome. Lancet 341:1664
Williams EA, Quinlan GJ, Goldstraw P, Gothard JW, Evans TW (1998) Postoperative lung injury and oxidative damage in patients undergoing pulmonary resection. Eur Respir J 11:1028–1034
Chiche JD, Canivet JL, Damas P, Joris J, Lamy M (1995) Inhaled nitric oxide for hemodynamic support after postpneumonectomy ARDS. Intensive Care Med 21:675–678
Rabkin DG, Sladen RN, DeMango A, Steinglass KM, Goldstein DJ (2001) Nitric oxide for the treatment of postpneumonectomy pulmonary edema. Ann Thorac Surg 72:272–274
Waldow T, Alexiou K, Witt W, Wagner FM, Kappert U, Knaut M, Matschke K (2004) Protection of lung tissue against ischemia/reperfusion injury by preconditioning with inhaled nitric oxide in an in situ pig model of normothermic pulmonary ischemia. Nitric Oxide 10:195–201
Thabut G, Brugiere O, Leseche G, Stern JB, Fradj K, Herve P, Jebrak G, Marty J, Fournier M, Mal H (2001) Preventive effect of inhaled nitric oxide and pentoxifylline on ischemia/reperfusion injury after lung transplantation. Transplantation 71:1295–1300
Meade MO, Granton JT, Matte-Martyn A, McRae K, Weaver B, Cripps P, Keshavjee SH (2003) A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med 167:1483–1489
Myles PS, Venema HR (1995) Avoidance of cardiopulmonary bypass during bilateral sequential lung transplantation using inhaled nitric oxide. J Cardiothorac Vasc Anesth 9:571–574
McIntyre RC Jr, Moore FA, Moore EE, Piedalue F, Haenel JS, Fullerton DA (1995) Inhaled nitric oxide variably improves oxygenation and pulmonary hypertension in patients with acute respiratory distress syndrome. J Trauma 39:418–425
Puybasset L, Rouby JJ, Mourgeon E, Cluzel P, Souhil Z, Law-Koune JD, Stewart T, Devilliers C, Lu Q, Roche S et al (1995) Factors influencing cardiopulmonary effects of inhaled nitric oxide in acute respiratory failure. Am J Respir Crit Care Med 152:318–328
Papazian L, Bregeon F, Gaillat F, Thirion X, Gainnier M, Gregoire R, Saux P, Gouin F, Jammes Y, Auffray JP (1998) Respective and combined effects of prone position and inhaled nitric oxide in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157:580–585
Gerlach H, Keh D, Semmerow A, Busch T, Lewandowski K, Pappert DM, Rossaint R, Falke KJ (2003) Dose-response characteristics during long-term inhalation of nitric oxide in patients with severe acute respiratory distress syndrome: a prospective, randomized, controlled study. Am J Respir Crit Care Med 167:1008–1015
Sokol J, Jacobs SE, Bohn D (2000) Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev:CD002787
Putensen C, Hormann C, Kleinsasser A, Putensen-Himmer G (1998) Cardiopulmonary effects of aerosolized prostaglandin E1 and nitric oxide inhalation in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 157:1743–1747
Walmrath D, Schneider T, Schermuly R, Olschewski H, Grimminger F, Seeger W (1996) Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med 153:991–996
Acknowledgements
Members of the Expert Recommendations Group are: Austria: Peter Germann, Roman Ullrich, Michael Grimm (Vienna); Belgium: Dirk Vlasselaers (Leuven); Denmark: Reinhold Helbo Jensen (Aarhus); France: Didier Payen, Anh Tuan Dinh-Xuan, Philippe Hervé (Orsay), Nicolas Dalibon, Suresnes; Jean-Jacques Lehot (Lyon); Germany: Konrad J. Falke, Udo Kaisers, Jörg Weimann (Berlin) Dietmar Schranz, Rainer Zimmermann (Giessen), Bernhard Zwissler (Frankfurt), Rolf Rossaint (Aachen); Konrad Reinhart (Jena); Bernd Müller (Marburg); Hungary: Janos Gal, Lajos Bogar (Pecs), Sandor Turi, László Vimláti (Szeged); Italy: Antonio Braschi (Pavia); Giorgio Della Rocca (Udine), Emilio D’Avino, Roberto Fumagalli, Luca Lorini (Bergamo), Marco Ranucci (Milan), Luigi Tritapepe (Rome); Poland: Andrzey Piotrowski (Lodz), Malgorzata Myc (Warsaw); Spain: Hector Litvan, Irene Rovina, Lluis Gallart, Antonio Artigas, Joan Ramon Masclans (Barcelona), Jose Maria Barrios (Madrid), Rosario Vicente (Valencia); Sweden: Claes Frostell, Lars Gustafsson (Danderyd), Ulla Westfeldt, Gunnar Sedin (Uppsala); Switzerland: Philippe Jolliet, Maurice Beghetti (Geneva), Marco Maggiorini (Zurich), Balthasar Eberle (Bern); United Kingdom: Duncan Macrae, Anita Szabo (London), Nandor Marczin (Harefield), Nigel R. Webster (Aberdeen); Norway: Jan Fredrik Bugge (Oslo); United States: Wolfgang Steudel (Denver). C.F. has participated in patent applications on the clinical use of iNO and has acted as a consultant to industry regarding the clinical use of iNO. K.J.F. is a member of the scientific advisory board of iNO Therapeutics U.S. and Europe. All authors received a reimbursement €2,500 for the time required to prepare their sections. C.F. and K.J.F. also have a relationship to these companies; C.F. receives consultancy payments from iNO Therapeutics of approx. €15,000 per year, and K.F. has received an average of U.S.$3,000 per year since the clinical introduction of iNO. Part of this money has been used for research and training purposes.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter Germann, Roman Ullrich: Both autors contributed equally in preparing and editing the manuscript.
This article refers to the article available at http://dx.doi.org/10.1007/s00134-005-2674-5
This conference was supported by an unrestricted grant from INO-Therapeutics.
Rights and permissions
About this article
Cite this article
Germann, P., Braschi, A., Della Rocca, G. et al. Inhaled nitric oxide therapy in adults: European expert recommendations. Intensive Care Med 31, 1029–1041 (2005). https://doi.org/10.1007/s00134-005-2675-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-005-2675-4