Abstract
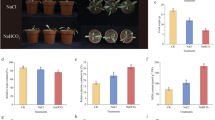
The differential transcript patterns of five antioxidant genes, four genes related to the ginsenoside pathway and five P450 genes related to defense mechanism were investigated in in vitro adventitious roots of Panax ginseng after exposure to two different concentrations of heavy metals for 7 days. PgSOD-1 and PgCAT transcription increased in a dose-dependent manner during the exposure to CuCl2, NiCl2, and CdCl2, while all other tested scavenging enzymes didn’t show significant increase during heavy metal exposure. Conversely, the mRNA transcripts of PgSQE, PgDDS were highly responsive to CuCl2 compared to NiCl2 exposure. However, the transcript profile of Pgβ-AS was highly induced upon NiCl2 treatment compared to CuCl2 and CdCl2 exposure. The expressions of PgCYP716A42, PgCYP71A50U, and PgCYP82C22 were regulated in similar manners, and all showed the highest transcript profile at 100 μM of CuCl2, CdCl2, and NiCl2 except PgCYP71D184, which showed the highest transcript level when subjected to 10 μM CuCl2 and NiCl2. Thus it may suggest that in P. ginseng heavy metal interaction on cell membrane induced expression of various defense related genes via jasmonic acid pathway and also possesses cross talk networks with other defense related pathways.





Similar content being viewed by others
References
Agrawal GK, Rakwal R, Jwa NS, Agrawal VP (2002) Effects of signaling molecules, protein phosphatase inhibitors and blast pathogen (Magnaporthe grisea) on the mRNA level of a rice (Oryza sativa L.) phospholipid hydroperoxide glutathione peroxidase (OsPHGPX) gene in seedling leaves. Gene 283:227–236
Alaousi-Sosse B, Genet P, Vimit-Dunand F, Toussaint M-L, Epron D, Badot P-M (2004) Effect of copper on growth in cucumber plants (Cucumis sativas) and its relationships with carbohydrate accumulation and changes in ion contents. Plant Sci 166:1213–1218
Ali MB, Thanh NT, Yu K-W, Hahn E-J, Paek K-Y, Lee HL (2005) Induction in the antioxidative systems and lipid peroxidation in suspension culture roots of Panax ginseng induced by oxygen in bioreactors. Plant Sci 169:833–841
Alissa EM, Bahijri SM, Lamb DJ, Ferns GA (2004) The effects of coadministration of dietary copper and zinc supplements on atherosclerosis, antioxidant enzymes and indices of lipid peroxidation in the cholesterol-fed rabbit. Int J Exp Pathol 85:265–275
Briskin DP (2000) Medicinal plants and phytomedicines. Linking plant biochemistry and physiology to human health. Plant Physiol 124:507–514. doi:10.1104/pp.124.2.507
Cakmak I (2005) The role of potassium in alleviating detrimental effects of abiotic stresses in plants. J Plant Nutr Soil Sci 168:521–530
Casella S, Frassinetti S, Lupi F, Squartini A (1998) Effect of cadminum, chromium and copper on symbiotic free living Rhizobium leguminosarium biovar trifolii. FEMS Microbiol Lett 49:343–347
Dada AOL, Hurtado FC, Czittrich N, Didierjean L, Schopfer C, Hertkorni N, Werck-Reichhart D, Ebel J (2001) Flavonoid 6-Hydroxylase from Soybean (Glycine max L.), a Novel Plant P-450 Monooxygenase. J Biol Chem 276:3
Devi BSR, Kim YJ, Sathiyamoorthy S, Khorolragchaa A, Gayathri S, Parvin S, Yang DU, Selvi SK, Lee OR, Lee S, Yang DC (2011) Classification and characterization of cytochrome P450 genes from Panax ginseng C.A. Meyer. Biochemistry (Mosc) 76(12):1347–1359
Devi BSR, Kim YJ, Selvi SK, Gayathri S, Altanzul K, Parvin S, Yang DC, Lee OR, Lee S, Yang DC (2012) Influence of potassium nitrate on antioxidant level and secondary metabolite genes under cold stress in Panax ginseng. Russ J Plant Phys 59(3):318–325
Foote CS, Wexler S (1964) Olefin oxidations with excited singlet molecular oxygen. J Am Chem Soc 86:3879–3880
Frank MR, Deyneka JM, Schuler MA (1996) Cloning of wound-lnduced cytochrome P450 monooxygenases expressed in pea. Plant Physiol 110:1035–1046
Gayathri S, Lee OK, Parvin S, Khorolragchaa A, Kim YJ, Yang DC (2011) Transcript profiling of antioxidant genes during biotic and abiotic stresses in Panax ginseng C.A. Meyer. Mol Biol Rep 38:2761–2769
Halliwell B, Gutteridge JMC (2006) Free radicals in biology and medicine, 4th edn. Clarendon Press, Oxford
Hu FX, Zhoong JJ (2007) Role of jasmonic acid in alteration of ginsenoside heterogenicity in elicited cell cultures of Panax notoginseng. J Biosci Bioeng 104:513–516. doi:10.1263/jbb.104.513
Hu FX, Zhoong JJ (2008) Jasmonic acid mediates gene transcription of ginsenoside biosynthesis in cell cultures of Panax notoginseng treated with chemically synthesized 2-hydroxyethyl jasmonate. Process Biochem 43:113–118. doi:10.1016/j.procbio.2007.10.010
Hu XY, Neill S, Cai WM, Tang Z (2003) Hydrogen peroxide and jasmonic acid mediate oligogalacturonic acid-induced saponin accumulation in suspension-cultured cells of Panax ginseng. Physiol Plant 118:414–421
Kane DO, Gill V, Boyd P, Burdon R (1996) Chilling, oxidative stress and antioxidant responses in Arabidopsis thaliana Callus. Planta 198:371–377
Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53:299–328. doi:10.1007/s10529-004-3183-2
Kim YS, Hahn EJ, Murthy HN, Pack KY (2004) Adventitious root growth and ginsenoside accumulation in Panax ginseng cultures as affected by methyl jasmonate. Biotechnol Lett 26:1619–1622. doi:10.1007/s10529-004-3183-2
Kuc J (1995) Phytoalexins, stress metabolism, and disease resistance in plants. Annu Rev Phytopathol 33:275–297
Lin Ch–Ch, Chen L-M, Liu Z-H (2005) Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci 168:855–861
Maksymiec W (2007) Signaling responses in plants to heavy metal stress. Acta Physiol Plant 9:177–187. doi:10.1007/s11738-007-0036-3
Maksymiec W, Krupa Z (2006a) Effects of methyl jasmonate and excess copper on root and leaf growth. Biol Plant (In press)
Maksymiec W, Krupa Z (2006b) The effects of short-term exposition top Cd, excess Cu ions and jasmonate on oxidative stress appearing in Arabidopsis thaliana. Environ Exp Bot 57:187–194
Mithofer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants for common signal. FEBS Lett 56:1–4
Mizukami H, Konoshima M, Tabata M (1977) Effect of nutritional factors on shikonin derivative formation in Lithospermum callus cultures. Phytochemistry 16:1183–1186
Mizutani M, Ohta D (2010) Diversification of P450 genes during land plant evolution. Annu Rev Plant Biol 61:291–315
Murashige T, Skoog F (1962) A revised medium for plant growth and bioassays with tobacco cultures. Physiol Plant 15:473–497
Ohlsson AB, Berglund T (1989) Effect of high MnSO4 levels on cardenolide accumulation by Digitalis lanata tissue cultures in light and darkness. J Plant Physiol 135:505–507
Pitzschke A, Hirt H (2006) Mitogen-activated protein kinases and reactive oxygen species signaling in plants. Plant Physiol 141:351–356
Ralston L, Kwon ST, Schoenbeck M, Ralston J, Schenk DJ, Coates RM, Chappell J (2001) Cloning, heterologous expression, and functional characterization of 5-epi-Aristolochene-1,3-Dihydroxylase from tobacco (Nicotiana tabacum). Arch Biochem 393(2):222–235
Sathiyamoorthy S, In JG, Gayathri S, Kim YJ, Yang DC (2009) Generation and gene ontology based analysis of expressed sequence tags (EST) from a Panax ginseng C.A. Meyer roots. Mol Biol Rep 46(7):932–939
Scutzendubel A, Polle A (2002) Plant responses to abiotic stresses: heavy-induced oxidative stress and protection by mycorrhization. J Exp Bot 53:1351–1365
Shibata S (2001) Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci 16:S28–S37
Sobkowiak E, Baszynski T (2003) Cadmium-induced changes in growth and cell cycle gene expression in suspension-culture cells of soybean. Plant Physiol Biochem 41:767–772
Sticher O (1998) Getting to the root of ginseng. ChemTech 28:26–32
Thimmaraju BN, Ravishankar GA (2004) In situ and ex situ adsorption and recovery of betalains from hairy root cultures of Beta vulgaris. Biotechnol Prog 20:777–785, PMID:15176882; doi:10.1021/bp0300570
Trejo-Tapia G, Jimenez-Aparicio A, Rodriguez-Monroy M, De Jesus-Sanchez A, Gutierrez-Lopez G (2001) Influence of cobalt and other microelements on the production of betalains and the growth of suspension cultures of Beta vulgaris. Plant Cell Tissue Org 67:19–23
Wang E, Wang R, DeParasis J, Loughrin JH, Gan S, Wagner GJ (2001) Suppression of a P450 hydroxylase gene in plant trichome glands enhances natural-product-based aphid resistance. Nat Biotechnol 19:371–374
Wang W, Zhao ZJ, Xu Y, Qian X, Zhong JJ (2006) Efficient induction of ginsenoside biosynthesis and alteration of ginsenoside heterogenecity in cell cultures of Panax notoginseng by using chemically synthesized 2-hydroxylethyl jasmonate. Appl Microbiol Biotechnol 70:298–307. doi:10.1007/s00253-005-0089-4
Woo S, Yum S, Park HS, Lee TK, Ryu JC (2009) Effects of heavy metals on antioxidants and stress-responsive gene expression in Japanese medeka (Oryzias javanicus). Comp Biochem Physiol 149:289–299
Zhao J, Davis L, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Acknowledgments
This research was supported by the Ministry of Knowledge Economy, Korea, under the Information Technology Research Center support program supervised by the National IT Industry Promotion Agency (NIPA-2011-C1090-1121-0003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balusamy, S.R.D., Kim, YJ., Rahimi, S. et al. Transcript Pattern of Cytochrome P450, Antioxidant and Ginsenoside Biosynthetic Pathway Genes Under Heavy Metal Stress in Panax ginseng Meyer. Bull Environ Contam Toxicol 90, 194–202 (2013). https://doi.org/10.1007/s00128-012-0891-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00128-012-0891-5