Abstract
Aims/hypothesis
Sodium–glucose co-transporter 2 (SGLT2) inhibitors (SGLT2i) are antihyperglycaemic drugs that protect the kidneys of individuals with type 2 diabetes mellitus. However, the underlying mechanisms mediating the renal benefits of SGLT2i are not fully understood. Considering the fuel switches that occur during therapeutic SGLT2 inhibition, we hypothesised that SGLT2i induce fasting-like and aestivation-like metabolic patterns, both of which contribute to the regulation of metabolic reprogramming in diabetic kidney disease (DKD).
Methods
Untargeted and targeted metabolomics assays were performed on plasma samples from participants with type 2 diabetes and kidney disease (n=35, 11 women) receiving canagliflozin (CANA) 100 mg/day at baseline and 12 week follow-up. Next, a systematic snapshot of the effect of CANA on key metabolites and pathways in the kidney was obtained using db/db mice. Moreover, the effects of glycine supplementation in db/db mice and human proximal tubular epithelial cells (human kidney-2 [HK-2]) cells were studied.
Results
Treatment of DKD patients with CANA for 12 weeks significantly reduced HbA1c from a median (interquartile range 25–75%) of 49.0 (44.0–57.0) mmol/mol (7.9%, [7.10–9.20%]) to 42.2 (39.7–47.7) mmol/mol (6.8%, [6.40–7.70%]), and reduced urinary albumin/creatinine ratio from 67.8 (45.9–159.0) mg/mmol to 47.0 (26.0–93.6) mg/mmol. The untargeted metabolomics assay showed downregulated glycolysis and upregulated fatty acid oxidation. The targeted metabolomics assay revealed significant upregulation of glycine. The kidneys of db/db mice undergo significant metabolic reprogramming, with changes in sugar, lipid and amino acid metabolism; CANA regulated the metabolic reprogramming in the kidneys of db/db mice. In particular, the pathways for glycine, serine and threonine metabolism, as well as the metabolite of glycine, were significantly upregulated in CANA-treated kidneys. Glycine supplementation ameliorated renal lesions in db/db mice by inhibiting food intake, improving insulin sensitivity and reducing blood glucose levels. Glycine supplementation improved apoptosis of human proximal tubule cells via the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway.
Conclusions/interpretation
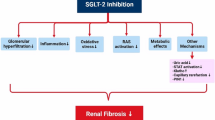
In conclusion, our study shows that CANA ameliorates DKD by inducing fasting-like and aestivation-like metabolic patterns. Furthermore, DKD was ameliorated by glycine supplementation, and the beneficial effects of glycine were probably due to the activation of the AMPK/mTOR pathway.
Graphical Abstract









Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- BAX:
-
BCL-2 associated X
- BCL-2:
-
B-cell lymphoma 2
- CANA:
-
Canagliflozin
- DEM:
-
Differentially expressed metabolite
- DKD:
-
Diabetic kidney disease
- FAO:
-
Fatty acid oxidation
- FBG:
-
fasting blood glucose
- GSH:
-
Glutathione
- GSSG:
-
Oxidised GSH
- H-CANA:
-
High-dose CANA group
- H-GLY:
-
High-concentration glycine group
- HK-2:
-
Human proximal tubular epithelial cells (human kidney-2)
- L-CANA:
-
Low-dose CANA group
- L-GLY:
-
Low-concentration glycine group
- MDA:
-
Malondialdehyde
- mTOR:
-
Mammalian target of rapamycin
- NC:
-
Normal control (group)
- OPLS-DA:
-
Orthogonal partial least-square discriminant analysis
- PAS:
-
Periodic acid–Schiff
- PCA:
-
Principal component analysis
- PIOG:
-
Pioglitazone treatment group
- SGLT2:
-
Sodium–glucose co-transporter 2
- SGLT2i:
-
Sodium–glucose co-transporter 2 inhibitors
- TEM:
-
Transmission electron microscopy
- UACR:
-
Urinary albumin/creatinine ratio
References
Alicic RZ, Johnson EJ, Tuttle KR (2018) SGLT2 Inhibition for the prevention and treatment of diabetic kidney disease: a review. Am J Kidney Dis 72:267–277. https://doi.org/10.1053/j.ajkd.2018.03.022
Koye DN, Magliano DJ, Nelson RG, Pavkov ME (2018) The global epidemiology of diabetes and kidney disease. Adv Chronic Kidney Dis 25:121–132. https://doi.org/10.1053/j.ackd.2017.10.011
Oellgaard J, Gæde P, Rossing P, Persson F, Parving HH, Pedersen O (2017) Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int 91:982–988. https://doi.org/10.1016/j.kint.2016.11.023
Afkarian M, Zelnick LR, Hall YN et al (2016) Clinical manifestations of kidney disease among US adults with diabetes, 1988–2014. JAMA 316:602–610. https://doi.org/10.1001/jama.2016.10924
Parving HH, de Zeeuw D, Cooper ME et al (2008) ACE gene polymorphism and losartan treatment in type 2 diabetic patients with nephropathy. J Am Soc Nephrol 19:771–779. https://doi.org/10.1681/ASN.2007050582
Chen Y, Fry BC, Layton AT (2016) Modeling glucose metabolism in the kidney. Bull Math Biol 78:1318–1336. https://doi.org/10.1007/s11538-016-0188-7
Bhargava P, Schnellmann RG (2017) Mitochondrial energetics in the kidney. Nat Rev Nephrol 13:629–646. https://doi.org/10.1038/nrneph.2017.107
Carvalho-Santos Z, Cardoso-Figueiredo R, Elias AP, Tastekin I, Baltazar C, Ribeiro C (2020) Cellular metabolic reprogramming controls sugar appetite in Drosophila. Nat Metab 2:958–973. https://doi.org/10.1038/s42255-020-0266-x
Zhang G, Darshi M, Sharma K (2018) The Warburg effect in diabetic kidney disease. Semin Nephrol 38:111–120. https://doi.org/10.1016/j.semnephrol.2018.01.002
Sas KM, Kayampilly P, Byun J et al (2016) Tissue-specific metabolic reprogramming drives nutrient flux in diabetic complications. JCI Insight 1:e86976. https://doi.org/10.1172/jci.insight.86976
Hussein H, Zaccardi F, Khunti K et al (2020) Efficacy and tolerability of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabetes Obes Metab 22:1035–1046. https://doi.org/10.1111/dom.14008
Chilton RJ (2020) Effects of sodium-glucose cotransporter-2 inhibitors on the cardiovascular and renal complications of type 2 diabetes. Diabetes Obes Metab 22:16–29. https://doi.org/10.1111/dom.13854
Vallon V, Verma S (2021) Effects of SGLT2 inhibitors on kidney and cardiovascular function. Annu Rev Physiol 83:503–528. https://doi.org/10.1146/annurev-physiol-031620-095920
DeFronzo RA, Reeves WB, Awad AS (2021) Pathophysiology of diabetic kidney disease: impact of SGLT2 inhibitors. Nat Rev Nephrol 17:319–334. https://doi.org/10.1038/s41581-021-00393-8
Adeghate EA, Kalász H, Al Jaberi S, Adeghate J, Tekes K (2021) Tackling type 2 diabetes-associated cardiovascular and renal comorbidities: a key challenge for drug development. Expert Opin Investig Drugs 30:85–93. https://doi.org/10.1080/13543784.2021.1865914
Artasensi A, Pedretti A, Vistoli G, Fumagalli L (2020) Type 2 diabetes mellitus: a review of multi-target drugs. Molecules 25:1987. https://doi.org/10.3390/molecules25081987
Gao YM, Feng ST, Wen Y, Tang TT, Wang B, Liu BC (2022) Cardiorenal protection of SGLT2 inhibitors–perspectives from metabolic reprogramming. EBioMedicine 83:104215. https://doi.org/10.1016/j.ebiom.2022.104215
Marton A, Kaneko T, Kovalik JP et al (2021) Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol 17:65–77. https://doi.org/10.1038/s41581-020-00350-x
Huynh C, Ryu J, Lee J, Inoki A, Inoki K (2023) Nutrient-sensing mTORC1 and AMPK pathways in chronic kidney diseases. Nat Rev Nephrol 19:102–122. https://doi.org/10.1038/s41581-022-00648-y
Kappel BA, Lehrke M, Schütt K et al (2017) Effect of empagliflozin on the metabolic signature of patients with type 2 diabetes mellitus and cardiovascular disease. Circulation 136:969–972. https://doi.org/10.1161/CIRCULATIONAHA.117.029166
Wong BW, Wang X, Zecchin A et al (2017) The role of fatty acid β-oxidation in lymphangiogenesis. Nature 542:49–54. https://doi.org/10.1038/nature21028
Knottnerus S, Bleeker JC, Wüst R et al (2018) Disorders of mitochondrial long-chain fatty acid oxidation and the carnitine shuttle. Rev Endocr Metab Disord 19:93–106. https://doi.org/10.1007/s11154-018-9448-1
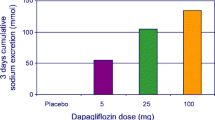
Mulder S, Hammarstedt A, Nagaraj SB et al (2020) A metabolomics-based molecular pathway analysis of how the sodium-glucose co-transporter-2 inhibitor dapagliflozin may slow kidney function decline in patients with diabetes. Diabetes Obes Metab 22:1157–1166. https://doi.org/10.1111/dom.14018
Ahmad AA, Draves SO, Rosca M (2021) Mitochondria in diabetic kidney disease. Cells 10:2945. https://doi.org/10.3390/cells10112945
Lieben L (2017) Diabetic nephropathy: lipid toxicity drives renal disease. Nat Rev Nephrol 13:194. https://doi.org/10.1038/nrneph.2017.22
Cai T, Ke Q, Fang Y et al (2020) Sodium-glucose cotransporter 2 inhibition suppresses HIF-1α-mediated metabolic switch from lipid oxidation to glycolysis in kidney tubule cells of diabetic mice. Cell Death Dis 11:390. https://doi.org/10.1038/nrneph.2017.22
Mora-Ortiz M, Nuñez Ramos P, Oregioni A, Claus SP (2019) NMR metabolomics identifies over 60 biomarkers associated with type ii diabetes impairment in db/db mice. Metabolomics 15:89. https://doi.org/10.1007/s11306-019-1548-8
Menni C, Fauman E, Erte I et al (2013) Biomarkers for type 2 diabetes and impaired fasting glucose using a nontargeted metabolomics approach. Diabetes 62:4270–4276. https://doi.org/10.2337/db13-0570
Lu YP, Zhang ZY, Wu HW et al (2022) SGLT2 inhibitors improve kidney function and morphology by regulating renal metabolic reprogramming in mice with diabetic kidney disease. J Transl Med 20:420. https://doi.org/10.1186/s12967-022-03629-8
Tamargo J, Segura J, Ruilope LM (2014) Diuretics in the treatment of hypertension. Part 2: loop diuretics and potassium-sparing agents. Expert Opin Pharmacother 15:605–621. https://doi.org/10.1517/14656566.2014.879117
Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ (2016) Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation 134:752–772. https://doi.org/10.1161/CIRCULATIONAHA.116.021887
McBean RL, Goldstein L (1970) Renal function during osmotic stress in the aquatic toad Xenopus laevis. Am J Physiol 219:1115–1123. https://doi.org/10.1152/ajplegacy.1970.219.4.1115
Ackrill P, Dixon JS, Green R, Thomas S (1970) Effects of prolonged saline exposure on water, sodium and urea transport and on electron-microscopical characteristics of the isolated urinary bladder of the toad Bufo bufo. J Physiol 210:73–85. https://doi.org/10.1113/jphysiol.1970.sp009196
Tomita I, Kume S, Sugahara S et al (2020) SGLT2 inhibition mediates protection from diabetic kidney disease by promoting ketone body-induced mTORC1 inhibition. Cell Metab 32:404-419.e6. https://doi.org/10.1016/j.cmet.2020.06.020
Kogot-Levin A, Hinden L, Riahi Y et al (2020) Proximal tubule mTORC1 is a central player in the pathophysiology of diabetic nephropathy and its correction by SGLT2 inhibitors. Cell Rep 32:107954. https://doi.org/10.1016/j.celrep.2020.107954
Kogot-Levin A, Riahi Y, Abramovich I et al (2023) Mapping the metabolic reprogramming induced by sodium-glucose cotransporter 2 inhibition. JCI Insight 8:e164296. https://doi.org/10.1172/jci.insight.164296
Takashina C, Tsujino I, Watanabe T et al (2016) Associations among the plasma amino acid profile, obesity, and glucose metabolism in Japanese adults with normal glucose tolerance. Nutr Metab (Lond) 13:5. https://doi.org/10.1186/s12986-015-0059-5
Glynn EL, Piner LW, Huffman KM et al (2015) Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 58:2324–2335. https://doi.org/10.1007/s00125-015-3705-6
Imenshahidi M, Hossenzadeh H (2022) Effects of glycine on metabolic syndrome components: a review. J Endocrinol Invest 45:927–939. https://doi.org/10.1007/s40618-021-01720-3
El-Hafidi M, Franco M, Ramírez AR et al (2018) Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxid Med Cell Longev 2018:2101562. https://doi.org/10.1155/2018/2101562
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Acknowledgements
The authors would like to thank C. Song (Zhengzhou University of Public Health, Henan, China) for helpful discussions. We also thank all patients who participated in this study and generously provided us with blood samples.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files.
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81974110, 82170839).
Authors’ relationships and activities
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
Contribution statement
MS, DC, YZ and GQ conceived and designed the research. MS, QW, FG, PD, YLiu, XM and GR contributed to the clinical trial and data collection. MS, DC, FW, WZ, TG, YLuo, XF and YS contributed to animal experiments and data collection. MS and FW contributed to cell experiments and data collection. MS and DC contributed to the analysis and interpretation of data. MS, DC and FW drafted the paper. WZ, TG, YLuo and XF revised the paper for important intellectual content. GQ revised the manuscript critically. All authors approved the final version of the manuscript prior to publication. YZ and GQ are the guarantors of this work, had full access to all of the study data and take responsibility for the integrity and accuracy of the data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mingwei Shao and Duo Chen contributed equally to this manuscript as joint first authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shao, M., Chen, D., Wang, Q. et al. Canagliflozin regulates metabolic reprogramming in diabetic kidney disease by inducing fasting-like and aestivation-like metabolic patterns. Diabetologia 67, 738–754 (2024). https://doi.org/10.1007/s00125-023-06078-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-023-06078-0