Abstract
Aims/hypothesis
Pancreatic ductal adenocarcinoma (PDAC) can cause type 3C diabetes, known as PDAC-associated diabetes mellitus (PDAC-DM), but the mechanism is unknown. This study aimed to reveal the mechanism.
Methods
PDAC lesions from patients with or without PDAC-DM (n = 4 in each group) were individually profiled for 23,512 mRNAs with microarrays. Bioinformatic analysis and in vivo and in vitro assays were then conducted.
Results
We determined that 2,778 genes were differentially expressed; over-representation of ten genes was validated with quantitative RT-PCR. The analysis of gene ontology showed that the differentially expressed secretory genes were related mainly to inflammation. High levels of a marker of inflammation (C-reactive protein [CRP]) and an inflammatory mediator (TNF super-family member 13 [TNFSF13]) were found in the serum of patients with PDAC-DM. After surgical resection of PDAC lesions, CRP and TNFSF13 levels significantly decreased (p < 0.01). Furthermore, we found that the levels of TNFSF13 in PDAC lesions and TNFSF13 and CRP in serum were significantly correlated with the diabetic status of patients with PDAC-DM (p < 0.01). Assays in vivo showed that after exposure to an inhibitor of inflammation (celecoxib), the fasting blood glucose level in the mouse model of PDAC-DM dramatically decreased from 6.9 ± 0.1 to 5.6 ± 0.1 mmol/l in 2–4 days (p < 0.01).
Conclusions/interpretation
We found that acute inflammation was involved in the pathogenesis of PDAC-DM. We contend that acute inflammation is a potential target for the diagnosis and treatment of PDAC-DM.
Similar content being viewed by others
Introduction
Pancreatic cancer leads to an estimated 227,000 deaths per year worldwide [1]. Some reports have noted an elevated risk of pancreatic cancer in individuals with diabetes mellitus [2]. Further analysis has shown that 74–88% of diabetes is ‘new onset’ in patients with pancreatic cancer [2]. Meanwhile, the risk of pancreatic cancer in individuals with new-onset diabetes mellitus is eightfold higher than in the general population [3]. Emerging evidence supports that the new-onset diabetes related to pancreatic cancer is an early manifestation of the cancer. Consequently, it is named as pancreatic ductal adenocarcinoma (PDAC)-associated diabetes mellitus (PDAC-DM), a type 3C diabetes [4]. The onset of PDAC-DM usually occurs 2–5 years before the diagnosis of PDAC and therefore its early recognition is vital in the screening for pancreatic cancer [2]. However, PDAC-DM cannot be differentiated from other types of diabetes by clinical signs and symptoms, and specific markers are urgently needed [2]. Several factors related to PDAC-DM have been identified, such as islet amyloid polypeptide and S-100A8 N-terminal peptide [2, 5, 6]. However, the role of islet amyloid polypeptide and other factors in the early diagnosis of PDAC-DM remains unclear.
Models show that factors secreted by pancreatic cancer cells can increase the insulin resistance of hepatocytes and muscle cells [7–9]. PDAC-DM starts when the pancreatic tumour is extremely small [2]. Evidence suggests that PDAC-DM is caused by factors secreted by the adenocarcinoma [2]. The secretory factors triggering PDAC-DM have been questioned [5, 10] and several factors such as S100A8 N-terminal peptide and hexokinase 2 have been identified [5]. However, global gene expression profiles of PDAC with PDAC-DM are not well elucidated.
In the present study, we explored the global gene expression profiles of PDAC lesions from patients with and without PDAC-DM. Bioinformatic analysis implied that the inflammation-related genes from gene ontology (GO) were over-represented in the lesions of patients with PDAC-DM. The correlations of a marker and a mediator of inflammation with the diabetic status of PDAC-DM patients were analysed, and the results demonstrated that inflammation was active in patients with PDAC-DM. Our experiments in vivo showed that celecoxib, an inhibitor of inflammation, could improve the fasting blood glucose (FBG) in the mouse model of PDAC-DM, which further supported the involvement of inflammation in the pathogenesis of PDAC-DM.
Methods
Clinical sample collection
Ethical approval of the study was granted by Sun Yat-sen Memorial Hospital Ethics Committee and written informed consent was obtained from each participant before the study started. Patients included in this study were registered between February 2010 and October 2014. The diagnoses of PDAC and chronic pancreatitis were supported by pathology results. Patients with PDAC, who met the diagnostic criteria of diabetes suggested by the American Diabetes Association before surgical resection of PDAC lesions, and who recovered from the diabetes after surgery, were diagnosed with PDAC-DM [4]. Patients with cachexia, autoimmune diseases, kidney failure, liver failure or hereditary disease were excluded. The control group comprised patients with PDAC or chronic pancreatitis with normal FBG, glucose tolerance and insulin resistance (see the electronic supplementary material [ESM] Methods), and none of the exclusion criteria (non-PDAC-DM, pancreatitis). The characteristics of these patients are provided in ESM Table 1. The surgically resected tissues were collected, incubated in the liquid nitrogen immediately and stored at −80°C for use. FBG (mmol/l) or a weighted FBG (mmol/l) was used to assess the diabetic status of patients with PDAC-DM (see ESM Methods for more details).
Quantitative RT-PCR and ELISA
Quantitative (q)RT-PCR and ELISA were performed as described previously [11] and the protocols are provided in ESM Methods. The primers used in this study are detailed in ESM Table 2.
Cell culture and mice
The protocols for cell culture and the preparation of conditioned medium are described in ESM Methods. Mice were purchased from and maintained at the Laboratory Animal Center of Sun Yat-sen University (Guangzhou, China) in a specific pathogen-free environment, and were cared for following the centre’s animal guidelines. Mice had continuous access to food and water unless fasting was required. After 6 h fasting, FBG levels in tail blood were analysed in triplicate using the Accu-Chek Active Glucometer (Roche Diagnostics, Mannheim, Germany). The mean value was recorded as FBG (mmol/l).
In vivo assay
Eighty wild-type female BALB/c mice, 6 weeks old, were randomised into four groups (n = 20 in each group). After the measurement of baseline FBG, all the mice were injected with unconditioned DMEM, conditioned medium from the human pancreatic cancer cell line MIA PaCa-2, conditioned medium from the human pancreatic carcinoma, epithelial-like cell line Panc1 and 0.9% (wt/vol.) NaCl solution every 2 days (subcutaneously 200 μl, and tail vein 100 μl), respectively. FBG was measured before each injection. After FBG had been stable for 3–5 days, ten mice from each group were additively treated with an inhibitor of inflammation (celecoxib, 45 mg kg−1 day−1, by gavage, once a day), and the other ten mice from the same group were additively treated with vehicle (lecithin-tylose slime, as the celecoxib control) until FBG was stable again. The administration of celecoxib/vehicle was then stopped and FBG was followed until it was stable. The mice were killed once FBG was stable.
Microarray analysis
The PDAC lesions from patients with or without PDAC-DM (n = 4 in each) were individually profiled and analysed for 23,512 mRNAs following the protocol in ESM Methods. The raw data has been released on the Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/index.cgi, ID GSE61166, accessed 11 October 2014). According to the subcellular location of encoded proteins, we further divided the differentially expressed genes (DEGs) into three groups: secretory genes, which encode secreted proteins; transmembrane genes, which encode transmembrane proteins; and intracellular genes, for which the encoded proteins are not secretory or transmembrane (ESM Methods and ESM Table 3).
Statistical analysis
The levels of FBG and TNF super-family member 13 (TNFSF13) in tissue and serum were analysed using the Student’s t test. The difference in levels of TNFSF13 and C-reactive protein (CRP) before and after surgery were analysed using a paired t test. Correlations were tested with Spearman correlation analysis; p < 0.05 was considered to be statistically significant. Statistical analysis was conducted using the SPSS (version 17.0, SPSS, Chicago, IL, USA).
Results
Global gene expression profiles indicate that PDAC triggers type 3C diabetes via inflammation
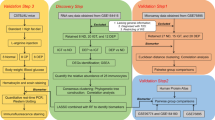
PDAC lesions in patients with or without PDAC-DM (patients A1–A4, PDAC-DM; patients B1–B4, PDAC without diabetes) were individually profiled with microarrays. The clinical characteristics of the eight patients are shown in Table 1. Data analysis was performed following the steps outlined in Fig. 1. Of the 16,551 genes, 951 genes were upregulated and 1,827 genes were downregulated in patients with PDAC-DM compared with PDAC patients without diabetes (p < 0.05, ESM Table 3). Ten DEGs were validated with qRT-PCR in the samples used for microarrays (Table 2); levels were consistent with the microarray results (Pearson’s test, p < 0.05). Of the DEGs, the number of secretory genes, transmembrane genes and intracellular genes were 488, 377 and 1,879, respectively (ESM Table 3). We then conducted the GO analysis on the differentially expressed secretory genes. The top 15 biological process items were mainly related to inflammation (Fig. 2). Interestingly, there was not any item directly associated with diabetes. Therefore, we hypothesised that PDAC triggered the pathogenesis of PDAC-DM in an indirect way related to inflammation.
Analysis workflow. RNA samples obtained from PDAC lesions with or without PDAC-DM (n = 4 in each group) were hybridised to Agilent microarrays. Expression data were analysed by Student’s t test to identify DEGs. Ten DEGs were validated by qRT-PCR and prediction of the subcellular location of the DEG-encoded proteins was performed using SignalP4.1 and SecretomeP2.0. GO and pathway analysis for secretory, transmembrane and intracellular genes were performed, respectively. Results of the bioinformatic analysis were validated by experiments in vitro and in vivo
A marker of inflammation (CRP) is correlated with PDAC-DM
We further detected the level of CRP, a marker of inflammation, in the serum of 26 PDAC-DM patients, 11 PDAC patients without diabetes and seven patients with chronic pancreatitis. We found that the pre-operative serum CRP level in patients with PDAC-DM was significantly higher than that in PDAC patients without diabetes and chronic pancreatitis patients (Fig. 3a, p < 0.01). After surgery, the serum CRP level in patients with PDAC-DM significantly decreased and was not different from that of PDAC patients without diabetes (Fig. 3a, p = 0.09).We also found that there was no significant difference in the CRP mRNA expression levels between the three groups (patients with PDAC-DM, PDAC patients without diabetes and patients with chronic pancreatitis) (p > 0.5, data not shown). In addition, the correlation between the CRP mRNA level and the pre-operative serum CRP level of the above 44 patients was evaluated, and no significant correlation was found (r = −0.15, p = 0.361, data not shown), which suggests that the elevated CRP in the serum of patients with PDAC-DM is not from PDAC lesions. In addition, we found that the difference in serum CRP was positively correlated with the difference in FBG in PDAC-DM patients before and after surgery (Fig. 3b; r = 0.828, p < 0.01). The above findings suggest that inflammation was active and was associated with the impaired FBG in patients with PDAC-DM.
Serum CRP levels and their correlation with FBG in patients with PDAC-associated diabetes. (a) Pre-operative (black bars) and postoperative (white bars) serum CRP levels; PDAC-DM, n = 26, patients A1–A26; non-PDAC-DM, n = 11, patients B1–B11; pancreatitis, n = 7, patients C1–C7. (b) The correlation between the difference in serum CRP and the difference in FBG (pre-operative values minus the postoperative values); n = 26, patients A1–A26. ** p < 0.01, Student’s t test; error bars show the standard deviation
An inflammatory mediator of inflammation (TNFSF13) is correlated with PDAC-DM
If inflammation is active in PDAC-DM, it must be accompanied by activation of inflammatory mediators, of which TNF-α is one of the most important [12]. However, TNF-α was not altered in our microarrays. Instead, we noticed that TNFSF13, which has high homology with TNF-α, was over-represented. First, we found that the pre-operative serum and tissue levels of TNFSF13 in patients with PDAC-DM were significantly higher than those of PDAC patients without diabetes and patients without chronic pancreatitis (Fig. 4a, b; p < 0.01). After surgery, serum TNFSF13 levels in patients with PDAC-DM decreased significantly (Fig. 4a; p < 0.01). Postoperative serum levels of TNFSF13 were not statistically different between PDAC patients with or without PDAC-DM (p = 0.16). In contrast, the pre-operative and postoperative serum TNFSF13 of chronic pancreatitis patients showed no obvious changes (p > 0.05). Next, we found that the serum TNFSF13 level was correlated with tissue TNFSF13 mRNA level in PDAC patients with PDAC-DM (Fig. 4c, r = 0.887, p < 0.01). The difference in FBG was significantly correlated with the difference in serum TNFSF13 before and after surgery (Fig. 4d, r = 0.890, p < 0.01); the difference in FBG was also correlated with the tissue TNFSF13 mRNA level (n = 21, r = 0.767, p < 0.01; data not shown). Our results indicated that the inflammatory mediator TNFSF13 was highly expressed in PDAC-DM and its expression correlated with the diabetic status of affected patients.
Serum and tissue TNFSF13 levels and their correlation with FBG. (a) Pre-operative (black bars) and postoperative (white bars) serum TNFSF13 levels; PDAC-DM, n = 26, patients A1–A26; non-PDAC-DM, n = 11, patients B1–B11; pancreatitis, n = 7, patients C1–C7. (b) mRNA expression of TNFSF13 in tissues; PDAC-DM, n = 21, patients A1–A4 and A10–A26; Non-PDAC-DM, n = 4, patients B1–B4; Pancreatitis, n = 4, patients C1–C4. (c) The correlation between pre-operative serum TNFSF13 level and TNFSF13 mRNA level in patients with PDAC-associated diabetes (n = 21, patients A1–A4 and A10–A26). (d) The correlation between the difference in TNFSF13 level and the difference in FBG post- vs pre-operatively in patients with PDAC-associated diabetes (n = 21, patients A1–A4 and A10–A26). ** p < 0.01, Student’s t test; error bars, standard deviation
In vivo assays show that acute inflammation has a role in PDAC-DM
To further assess the role of inflammation, we conducted assays in vivo using a mouse model of PDAC-DM (Fig. 5a, b). Baseline FBG levels for each group were statistically the same (one-way ANOVA, F = 0.058). Over a median of 5 days (ranging from 3 to 8 days), FBG levels in mice treated with conditioned medium from Panc1 (Panc1 mice) and MIA PaCa-2 (MIA mice) cells increased from 5.0 ± 0.0 mmol/l to 6.9 ± 0.1 mmol/l and 7.0 ± 0.1 mmol/l, respectively. In contrast, FBG levels in mice treated with unconditioned DMEM or NaCl solution were not changed (4.7 ± 0.1 mmol/l and 5.0 ± 0.3 mmol/l, respectively). After additively treating with an inhibitor of inflammation (celecoxib, 45 mg kg−1 day−1), the FBG of Panc1 mice and MIA mice dramatically decreased to 5.6 ± 0.1 mmol/l and 5.7 ± 0.2 mmol/l within approximately 2 days (ranging from 2 to 4 days), respectively. Treatment of celecoxib was then stopped, and the FBG levels rebounded to 6.9 ± 0.4 and 7.1 ± 0.1 mmol/l in approximately 3 days, respectively. Nevertheless, the influence of vehicle (celecoxib control) on FBG of Panc1 and MIA mice, and the influence of celecoxib and vehicle on FBG of mice treated with DMEM and NaCl solution was limited. The assays showed that the conditioned medium of PDAC cell lines was able to elevate the FBG level of mice in about 5 days and inhibition of inflammation with celecoxib rapidly reduced the elevated FBG, implying that acute inflammation is involved in PDAC-DM.
In vivo model to explore the role of inflammation in PDAC-associated diabetes. (a) Work flow of the experiment. (b) FBG of the mice: circles, mice were treated with conditioned Panc1 medium and then additively treated with celecoxib; diamonds, mice were treated with conditioned Panc1 medium and then additively treated with vehicle (celecoxib control); squares, mice were treated with unconditioned medium and then additively treated with celecoxib; triangles, mice were treated with unconditioned medium and then additively treated with vehicle. Conditioned/unconditioned medium was used on the day marked ‘Medium’. Celecoxib/vehicle was used on the day marked ‘Celecoxib’. Celecoxib/vehicle was stopped on the day marked ‘Stop celecoxib’
Numerous inflammatory mediators and signalling pathways are over-represented in PDAC-DM
We searched for inflammatory mediators and their regulatory pathways over-represented in lesions of PDAC-DM. Inflammatory mediators have been manually classified into seven groups (Table 3) [13–15]. We found that 238 differentially expressed secretory genes were inflammatory mediators and the number was about half that of the differentially expressed secretory genes (ESM Table 4). We also found that the over-represented GO items of transmembrane DEGs were mainly associated with signal transduction, bacterial infection and RNA/protein metabolism (Fig. 6a). In the analysis of intracellular genes, we discovered that the predominantly upregulated pathway was Ras–mitogen-activated protein kinase (MAPK) signalling (Fig. 6b).
Discussion
The development of PDAC-DM is a promising clue for the early detection of pancreatic cancer, but how PDAC causes diabetes is largely unknown. We analysed global gene expression profiles of PDAC lesions from patients with and without PDAC-DM. Analysis of clinical and experimental data indicated that acute inflammation was involved.
Numerous genes were found to be differentially expressed on bioinformatic analysis of the gene expression profiles, consistent with former reports [16, 17]. We then extracted secretory genes and did GO analysis. Surprisingly, no GO item was directly related to diabetes. Instead, we found that inflammation-related items were significantly over-represented. We therefore hypothesised that secretory factors triggered type 3C diabetes mainly via indirect ways related to inflammation, even though PDAC is able to induce diabetes directly [2]. Although it has been reported that the FBG level of immunodeficient mice is increased by conditioned medium from a PDAC cell line (MIA PaCa-2), indicating that adaptive immunity is not necessary for the pathogenesis of PDAC-DM [9], the role of inflammation has not been studied.
Activation of inflammation was accompanied by an elevation in levels of serum markers and mediators. In the present study, we found that levels of the serum marker of acute inflammation (CRP) and an inflammatory mediator (TNFSF13) were significantly higher in patients with PDAC-DM than in those without diabetes and in pancreatitis patients. Though the serum levels of CRP and TNFSF13 in patients with PDAC-DM were not directly compared with those of healthy individuals in our study, the serum CRP and TNFSF13 levels in patients with chronic pancreatitis were shown to be slightly higher and the same as those of healthy individuals, respectively [18–20]. Our data demonstrated similar levels of CRP mRNA expression in tissues from patients with and without PDAC-DM, and in patients with chronic pancreatitis, excluding the possibility that PDAC lesions are the source of the elevated CRP in the serum of patients with PDAC-DM. In contrast, TNFSF13 was significantly increased in the serum of the patients with PDAC-DM, and the serum levels correlated with TNFSF13 tissue levels in PDAC lesions of the affected patients. We contend that inflammation is active in PDAC-DM and that TNFSF13 may be a mediator.
In addition, our in vivo experiment suggested that the inflammation activated in PDAC-DM was acute. In as short a time as 3 days, the mouse FBG levels were elevated significantly by conditioned medium from PDAC cell lines. After celecoxib administration, the FBG elevated by the conditioned medium dramatically decreased in 2–4 days. This implied that PDAC caused PDAC-associated diabetes in an acute manner. Celecoxib selectively inhibits cyclooxygenase-2, which is closely associated with a variety of inflammatory mediators. Inhibition of cyclooxygenase-2 with celecoxib has been proven to reduce the signs and symptoms of inflammation in several disease models [21].
Collectively, our data indicate that acute inflammation is involved in the pathogenesis of PDAC-DM. However, it is largely unknown how acute inflammation acts in PDAC-associated diabetes. The inflammatory mediators secreted by PDAC cells are not well characterised. We found that TNFSF13 in PDAC lesions was correlated with the diabetic status of patients with PDAC-associated diabetes, but whether the elevated serum TNFSF13 is secreted by PDAC cells is uncertain. Factors such as adrenomedullin and S1009A can be secreted by PDAC cells and have been suggested to play a role in PDAC-DM, but whether these cause the associated acute inflammation has not been studied [2]. Second, chronic inflammation leads to type 2 diabetes by recruiting macrophages [22], but the targets of the inflammatory mediators secreted by PDAC cells are largely undefined. Our bioinformatic analysis indicates that the Ras signalling pathway is over-represented in PDAC-DM and previous reports indicate that PDAC-associated diabetes is related to v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog (Kras) mutations [5, 23], but the role of Ras and other regulatory signalling pathways in PDAC-associated diabetes needs to be confirmed.
Acute inflammation is a potential target for the treatment and early recognition of PDAC-associated diabetes. Hyperglycaemia of PDAC-associated diabetes is difficult to control [24], but FBG levels in the mouse model of PDAC-DM decreased after oral administration of celecoxib in our study. Whether celecoxib can decrease the FBG of patients with PDAC-associated diabetes is worth researching. Screening for pancreatic cancer by using PDAC-associated diabetes as a filter is not sufficiently effective because we cannot distinguish PDAC-DM from type 2 diabetes at present. Inflammation is also seen in type 2 diabetes, but inhibition of inflammation only moderately decreases the FBG of these patients [12]. Our study shows that the administration of celecoxib may significantly decrease FBG in PDAC-associated diabetes. It potentially provides a new approach for the early recognition of PDAC-DM, which may lead to earlier diagnosis of pancreatic cancer.
In conclusion, our study shows that acute inflammation was involved in the pathogenesis of PDAC-DM. We contend that acute inflammation is a potential target for treatment and diagnosis of PDAC-DM.
Abbreviations
- CRP:
-
C-reactive protein
- DEG:
-
Differentially expressed gene
- FBG:
-
Fasting blood glucose
- GO:
-
Gene ontology
- non-PDAC-DM:
-
PDAC without diabetes
- PDAC:
-
Pancreatic ductal adenocarcinoma
- PDAC-DM:
-
PDAC-associated diabetes mellitus
- qRT-PCR:
-
Quantitative RT-PCR
- TNFSF13:
-
TNF super-family member 13
References
Vincent A, Herman J, Schulick R, Hruban RH, Goggins M (2011) Pancreatic cancer. Lancet 378:607–620
Pannala R, Basu A, Petersen GM, Chari ST (2009) New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol 10:88–95
Chari ST, Leibson CL, Rabe KG, Ransom J, de Andrade M, Petersen GM (2005) Probability of pancreatic cancer following diabetes: a population-based study. Gastroenterology 129:504–511
American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36(Suppl 1):S67–S74
Li J, Cao G, Ma Q, Liu H, Li W, Han L (2012) The bidirectional interaction between pancreatic cancer and diabetes. World J Surg Oncol 10:171
Padoan A, Seraglia R, Basso D et al (2013) Usefulness of MALDI-TOF/MS identification of low-MW fragments in sera for the differential diagnosis of pancreatic cancer. Pancreas 42:622–632
Basso D, Millino C, Greco E et al (2004) Altered glucose metabolism and proteolysis in pancreatic cancer cell conditioned myoblasts: searching for a gene expression pattern with a microarray analysis of 5000 skeletal muscle genes. Gut 53:1159–1166
Valerio A, Basso D, Brigato L et al (1999) Glucose metabolic alterations in isolated and perfused rat hepatocytes induced by pancreatic cancer conditioned medium: a low molecular weight factor possibly involved. Biochem Biophys Res Commun 257:622–628
Basso D, Brigato L, Veronesi A, Panozzo MP, Amadori A, Plebani M (1995) The pancreatic cancer cell line MIA PaCa2 produces one or more factors able to induce hyperglycemia in SCID mice. Anticancer Res 15:2585–2588
Chou KC (2001) Prediction of protein cellular attributes using pseudo-amino acid composition. Proteins 43:246–255
Zhang HL, Hassan MY, Zheng XY et al (2012) Attenuated EAN in TNF-alpha deficient mice is associated with an altered balance of M1/M2 macrophages. PLoS One 7:e38157
Donath MY (2014) Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat Rev Drug Discov 13:465–476
Piya MK, McTernan PG, Kumar S (2013) Adipokine inflammation and insulin resistance: the role of glucose, lipids and endotoxin. J Endocrinol 216:T1–T15
Ma K, Jin X, Liang X, Zhao Q, Zhang X (2012) Inflammatory mediators involved in the progression of the metabolic syndrome. Diabetes Metab Res Rev 28:388–394
Muoio DM, Newgard CB (2008) Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 9:193–205
Wang WS, Liu XH, Liu LX, Jin DY, Yang PY, Wang XL (2013) Identification of proteins implicated in the development of pancreatic cancer-associated diabetes mellitus by iTRAQ-based quantitative proteomics. J Proteome 84:52–60
Huang H, Dong X, Kang MX et al (2010) Novel blood biomarkers of pancreatic cancer-associated diabetes mellitus identified by peripheral blood-based gene expression profiles. Am J Gastroenterol 105:1661–1669
Zhang P, Zou M, Wen X et al (2014) Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer 134:2646–2655
Mroczko B, Groblewska M, Gryko M, Kedra B, Szmitkowski M (2010) Diagnostic usefulness of serum interleukin 6 (IL-6) and C-reactive protein (CRP) in the differentiation between pancreatic cancer and chronic pancreatitis. J Clin Lab Anal 24:256–261
Wang F, Chen L, Ding W et al (2011) Serum APRIL, a potential tumor marker in pancreatic cancer. Clin Chem Lab Med 49:1715–1719
Turini ME, DuBois RN (2002) Cyclooxygenase-2: a therapeutic target. Annu Rev Med 53:35–57
Donath MY (2013) Targeting inflammation in the treatment of type 2 diabetes. Diabetes Obes Metab 15(Suppl 3):193–196
Liu E, Thant AA, Kikkawa F et al (2000) The Ras-mitogen-activated protein kinase pathway is critical for the activation of matrix metalloproteinase secretion and the invasiveness in v-crk-transformed 3Y1. Cancer Res 60:2361–2364
Ewald N, Bretzel RG (2013) Diabetes mellitus secondary to pancreatic diseases (type 3c)–are we neglecting an important disease? Eur J Intern Med 24:203–206
Acknowledgements
Y. Yang (University of Pennsylvania) and D. Li (University of Texas MD Anderson Cancer Center) provided numerous constructive suggestions about the manuscript and we are so very grateful for everything they did. We are particularly indebted to S. Lian, Y. Yang and Z. Dai (Department of Electronics, Sun Yat-Sen University) for their help with the bioinformatics. We thank X. Wu, X. Zhang and N. Zhou for their assistance preparing the samples. We also appreciate the help from Z. Fu, L. Ye and C. Chen (Sun Yat-sen Memorial Hospital) with the patient data collection.
Funding
This research was supported by the Specialized Research Fund for the Doctoral Program of Higher Education (grant number 20090171110067, www.cutech.edu.cn/cn/kyjj/gdxxbsdkyjj/A010301index_1.htm, the National Natural Science Foundation of Guangdong Province (grant number S2012010008934, http://pro.gdstc.gov.cn/egrantweb/) and the National Natural Science Foundation of China (grant number 81000889, www.nsfc.gov.cn/).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
WG, YZ, QZ, LT, ZL, YS and XZ contributed to the conception of the experiments and drafting the article. QLin, MY, SZ and HY contributed to the acquisition of data and the drafting. WG, BZ, QLi, JZ, YL and HH contributed to analysis and interpretation of data and writing the manuscript. HZ and XH helped to analyse the data, draft the article and provided some reagents. ZL, XD and RC revised the manuscript critically for important intellectual content and designed the experiments. All authors gave a final approval of the version to be published with a signed statement. RC is the guarantor of this work.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Wenchao Gao, Yu Zhou and Qingyan Li contributed equally to this work
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods
(PDF 242 kb)
ESM Table 1
(PDF 162 kb)
ESM Table 2
(PDF 181 kb)
ESM Table 3
(PDF 1883 kb)
ESM Table 4
(PDF 124 kb)
Rights and permissions
About this article
Cite this article
Gao, W., Zhou, Y., Li, Q. et al. Analysis of global gene expression profiles suggests a role of acute inflammation in type 3C diabetes mellitus caused by pancreatic ductal adenocarcinoma. Diabetologia 58, 835–844 (2015). https://doi.org/10.1007/s00125-014-3481-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3481-8