Abstract
Aims/hypothesis
South Asians have a higher risk of developing type 2 diabetes than Europeans. The underlying cause of this excess risk is still poorly understood but might be related to differences in the regulation of energy/nutrient-sensing pathways in metabolic tissues and subsequent changes in whole-body substrate metabolism. In this study, we investigated the whole-body and skeletal muscle metabolic adaptations to short-term energy restriction in South Asian and European volunteers.
Methods
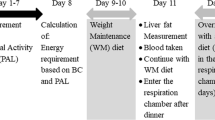
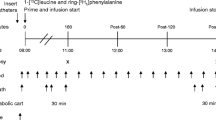
Twenty-four middle-aged overweight South Asian and European men underwent a two-step hyperinsulinaemic–euglycaemic clamp, with skeletal muscle biopsies and indirect calorimetry before and after an 8 day diet very low in energy (very low calorie diet [VLCD]). Abdominal fat distribution and hepatic triacylglycerol content were assessed using MRI and MR spectroscopy.
Results
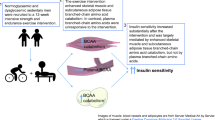
South Asian men had higher hepatic triacylglycerol content than European men, and exhibited elevated clamp insulin levels that probably reflect a lower insulin clearance rate. Despite higher insulin levels, endogenous glucose production rate was similar and glucose disposal rate (Rd) and nonoxidative glucose disposal rate (NOGD) were significantly lower in South Asian than European men, indicating impaired whole-body insulin sensitivity. Energy restriction decreased abdominal fat mass and hepatic triacylglycerol content in both groups. However, the shift induced by energy restriction from glucose towards lipid oxidation observed in European men was impaired in South Asian men, indicating whole-body metabolic inflexibility. Remarkably, although energy restriction improved hepatic insulin sensitivity in both groups, Rd improved only in South Asian men owing to higher NOGD. At the molecular level, an increase in insulin-induced activation of the skeletal muscle mTOR pathway was found in South Asian men, showing that skeletal muscle energy/nutrient-sensing pathways were differentially affected by energy restriction.
Conclusions/interpretation
We conclude that South Asian men exhibit a different metabolic adaptation to short-term energy restriction than European men.
Trial registration: Dutch trial registry (www.trialregister.nl), trial number NTR 2473.





Similar content being viewed by others
Abbreviations
- 4EBP1:
-
Eukaryotic translation initiation factor 4E-binding protein 1
- ACC:
-
Acetyl-CoA carboxylase
- AMPK:
-
AMP-activated protein kinase
- AS160:
-
Akt substrate of 160 kDa
- EGP:
-
Endogenous glucose production
- ERK:
-
Extracellular signal-regulated kinase
- GS:
-
Glycogen synthase
- GSK3:
-
Glycogen synthase kinase-3
- HIR:
-
Hepatic insulin resistance
- IQR:
-
Interquartile range
- IRβ:
-
Insulin receptor β
- IRS1:
-
Insulin receptor substrate 1
- LBM:
-
Lean body mass
- MCRi :
-
Metabolic clearance rate of insulin
- mtDNA:
-
Mitochondrial DNA
- mTOR:
-
Mammalian target of rapamycin
- mTORC1:
-
Mammalian target of rapamycin complex 1
- nDNA:
-
Nuclear DNA
- NEFA:
-
Nonesterified fatty acid
- NOGD:
-
Nonoxidative glucose disposal
- PKB:
-
Protein kinase B
- PPARα:
-
Peroxisome proliferator-activated receptor α
- PRAS40:
-
Proline-rich Akt substrate of 40 kDa
- Rd :
-
Rate of glucose disposal
- REE:
-
Resting energy expenditure
- RQ:
-
Respiratory quotient
- S6K1:
-
Ribosomal protein S6 kinase β1
- TSC2:
-
Tuberous sclerosis complex 2
- VLCD:
-
Very low calorie diet
References
National Task Force on the Prevention and Treatment of Obesity (2000) Overweight, obesity, and health risk. Arch Intern Med 160:898–904
Mindell J, Zaninotto P (2006) Cardiovascular disease and diabetes. In: Sproston K, Mindell J (eds) Health survey for England 2004. Volume 1. The health of minority ethnic groups, 1st edn. The Information Centre, Leeds, pp 63–94
Bindraban NR, van Valkengoed IG, Mairuhu G et al (2008) Prevalence of diabetes mellitus and the performance of a risk score among Hindustani Surinamese, African Surinamese and ethnic Dutch: a cross-sectional population-based study. BMC Public Health 8:271–280
Chiu M, Austin PC, Manuel DG, Shah BR, Tu JV (2011) Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care 34:1741–1748
Whiting DR, Guariguata L, Weil C, Shaw J (2011) IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 94:311–321
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Haruta T, Uno T, Kawahara J et al (2000) A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 14:783–794
Ricoult SJ, Manning BD (2013) The multifaceted role of mTORC1 in the control of lipid metabolism. EMBO Rep 14:242–251
Takano A, Usui I, Haruta T et al (2001) Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol 21:5050–5062
Um SH, Frigerio F, Watanabe M et al (2004) Absence of S6K1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 431:200–205
Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 40:310–322
Gwinn DM, Shackelford DB, Egan DF et al (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30:214–226
Inoki K, Zhu T, Guan KL (2003) TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590
Hammer S, van der Meer RW, Lamb HJ et al (2008) Short-term flexibility of myocardial triglycerides and diastolic function in patients with type 2 diabetes mellitus. Am J Physiol Endocrinol Metab 295:E714–E718
van der Meer RW, Hammer S, Lamb HJ et al (2008) Effects of short-term high-fat, high-energy diet on hepatic and myocardial triglyceride content in healthy men. J Clin Endocrinol Metab 93:2702–2708
Bakker LE, van Schinkel LD, Guigas B et al (2014) A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes 63:248–258
Corpeleijn E, Saris WH, Blaak EE (2009) Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle. Obes Rev 10:178–193
Szuhai K, Ouweland J, Dirks R et al (2001) Simultaneous A8344G heteroplasmy and mitochondrial DNA copy number quantification in myoclonus epilepsy and ragged-red fibers (MERRF) syndrome by a multiplex molecular beacon based real-time fluorescence PCR. Nucleic Acids Res 29:E13
Wijngaarden MA, van der Zon GC, Willems van Dijk KW, Pijl H, Guigas B (2013) Effects of prolonged fasting on AMPK signaling, gene expression and mitochondrial respiratory-chain content in skeletal muscle from lean and obese individuals. Am J Physiol Endocrinol Metab 304:E1012–E1021
Seppala-Lindroos A, Vehkavaara S, Hakkinen AM et al (2002) Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 87:3023–3028
Anand SS, Tarnopolsky MA, Rashid S et al (2011) Adipocyte hypertrophy, fatty liver and metabolic risk factors in South Asians: the Molecular Study of Health and Risk in Ethnic Groups (mol-SHARE). PLoS One 6:e22112
Petersen KF, Dufour S, Feng J et al (2006) Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci U S A 103:18273–18277
Shulman GI, Rothman DL, Jue T, Stein P, DeFronzo RA, Shulman RG (1990) Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med 322:223–228
Jensen J, Ruge T, Lai YC, Svensson MK, Eriksson JW (2011) Effects of adrenaline on whole-body glucose metabolism and insulin-mediated regulation of glycogen synthase and PKB phosphorylation in human skeletal muscle. Metabolism 60:215–226
Skurat AV, Dietrich AD (2004) Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem 279:2490–2498
Wilson WA, Skurat AV, Probst B, de Paoli-Roach A, Roach PJ, Rutter J (2005) Control of mammalian glycogen synthase by PAS kinase. Proc Natl Acad Sci U S A 102:16596–16601
van der Meer RW, Hammer S, Smit JW et al (2007) Short-term caloric restriction induces accumulation of myocardial triglycerides and decreases left ventricular diastolic function in healthy subjects. Diabetes 56:2849–2853
Christiansen MP, Linfoot PA, Neese RA, Hellerstein MK (2000) Effect of dietary energy restriction on glucose production and substrate utilization in type 2 diabetes. Diabetes 49:1691–1699
Jazet IM, Pijl H, Frolich M, Romijn JA, Meinders AE (2005) Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism 54:705–712
Markovic TP, Jenkins AB, Campbell LV, Furler SM, Kraegen EW, Chisholm DJ (1998) The determinants of glycemic responses to diet restriction and weight loss in obesity and NIDDM. Diabetes Care 21:687–694
Galgani JE, Heilbronn LK, Azuma K et al (2008) Metabolic flexibility in response to glucose is not impaired in people with type 2 diabetes after controlling for glucose disposal rate. Diabetes 57:841–845
van de Weijer T, Sparks LM, Phielix E et al (2013) Relationships between mitochondrial function and metabolic flexibility in type 2 diabetes mellitus. PLoS One 8:e51648
Inoki K, Li Y, Xu T, Guan KL (2003) Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev 17:1829–1834
Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4:648–657
Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP (2005) Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell 121:179–193
Carriere A, Romeo Y, Acosta-Jaquez HA et al (2011) ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1). J Biol Chem 286:567–577
Vander Haar E, Lee SI, Bandhakavi S, Griffin TJ, Kim DH (2007) Insulin signalling to mTOR mediated by the Akt/PKB substrate PRAS40. Nat Cell Biol 9:316–323
Rajkhowa M, Brett S, Cuthbertson DJ et al (2009) Insulin resistance in polycystic ovary syndrome is associated with defective regulation of ERK1/2 by insulin in skeletal muscle in vivo. Biochem J 418:665–671
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM (2010) mTORC1 controls fasting-induced ketogenesis and its modulation by ageing. Nature 468:1100–1104
Rakhshandehroo M, Knoch B, Muller M, Kersten S (2010) Peroxisome proliferator-activated receptor alpha target genes. PPAR Res 2010:
Blaak EE, Wagenmakers AJ, Glatz JF et al (2000) Plasma FFA utilization and fatty acid-binding protein content are diminished in type 2 diabetic muscle. Am J Physiol Endocrinol Metab 279:E146–E154
Kelley DE, Goodpaster B, Wing RR, Simoneau JA (1999) Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol 277:E1130–E1141
Khamzina L, Veilleux A, Bergeron S, Marette A (2005) Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology 146:1473–1481
Tremblay F, Brule S, Hee US et al (2007) Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci U S A 104:14056–14061
Copps KD, White MF (2012) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55:2565–2582
Hardie DG (2011) Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc Nutr Soc 70:92–99
Richter EA, Hargreaves M (2013) Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 93:993–1017
Geraghty KM, Chen S, Harthill JE et al (2007) Regulation of multisite phosphorylation and 14-3-3 binding of AS160 in response to IGF-1, EGF, PMA and AICAR. Biochem J 407:231–241
Karlsson HK, Chibalin AV, Koistinen HA et al (2009) Kinetics of GLUT4 trafficking in rat and human skeletal muscle. Diabetes 58:847–854
Hunter RW, Treebak JT, Wojtaszewski JF, Sakamoto K (2011) Molecular mechanism by which AMP-activated protein kinase activation promotes glycogen accumulation in muscle. Diabetes 60:766–774
Acknowledgements
We are greatly indebted to E. J. M. Ladan-Eygenraam (Leiden University Medical Centre, Leiden, the Netherlands) for her technical assistance during the study.
Funding
We thank Roba Metals B. V. IJsselstein (Utrecht, the Netherlands) for financial support. Funding by the Netherlands Heart Foundation (Project UL 2009-4548) is gratefully acknowledged (The Hague, the Netherlands). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
All authors have read and approved the final version of the manuscript, and meet all three conditions as stated by the International Committee of Medical Journal Editors uniform requirements for manuscripts submitted to medical journals. LEHB contributed to the acquisition, analysis and interpretation of all data and drafted the manuscript. BG contributed to the acquisition, analysis and interpretation of skeletal muscle data and reviewed the manuscript. LDS contributed to the acquisition, analysis and interpretation of MR data and reviewed the manuscript. GCMZ contributed to the acquisition of skeletal muscle data and reviewed the manuscript. TCMS contributed to the acquisition of clamp data and reviewed the manuscript. JBK provided the statistical model, contributed to the analysis of data and reviewed the manuscript. JTJ contributed to the design of the MR protocol, contributed to the acquisition and interpretation of MR data and reviewed the manuscript. HJL contributed to the analysis and interpretation of MR data and reviewed the manuscript. JWAS contributed to the conception and design of the MR protocol, contributed to the analysis and interpretation of data and reviewed the manuscript. HP contributed to the analysis and interpretation of data and reviewed the manuscript. AEM contributed to the conception and design of the study protocol, contributed to the analysis and interpretation of all data and reviewed the manuscript. IMJ contributed to the conception and design of the protocol, contributed to the acquisition, analysis and interpretation of all data and reviewed the manuscript.
LEHB and IMJ are the guarantors of this work and, as such, have full access to all the data generated in the framework of the study and take responsibility for their integrity and the accuracy of their analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 354 kb)
ESM Table 1
(PDF 333 kb)
Rights and permissions
About this article
Cite this article
Bakker, L.E.H., Guigas, B., van Schinkel, L.D. et al. Middle-aged overweight South Asian men exhibit a different metabolic adaptation to short-term energy restriction compared with Europeans. Diabetologia 58, 165–177 (2015). https://doi.org/10.1007/s00125-014-3408-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3408-4