Abstract
Aims/hypothesis
Obesity and diabetes increase the risk of developing cardiovascular diseases and heart failure. These metabolic disorders are generally reflected by natriuretic peptide system deficiency. Since brain natriuretic peptide (BNP) is known to influence metabolism and cardioprotection, we investigated the effect of chronic exogenous BNP treatment on adverse myocardial consequences related to obesity and diabetes.
Methods
Ten-week-old C57BL/KsJ-db/db obese diabetic mice (db/db) and their lean control littermates (db/+) were treated with BNP (0.6 μg kg−1 h−1) or saline for 12 weeks (n = 10/group). Serial blood and tomography analysis were performed. Cardiac function was determined by echocardiography, and biochemical and histological heart and fat analyses were also performed.
Results
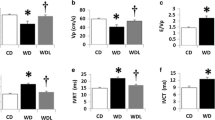
BNP treatment resulted in an average increase in plasma BNP levels of 70 pg/ml. An improvement in the metabolic profile of db/db mice was observed, including a reduction in fat content, increased insulin sensitivity, improved glucose tolerance and lower blood glucose, despite increased food intake. db/db mice receiving saline displayed both early systolic and diastolic dysfunction, whereas these functional changes were prevented by BNP treatment. The cardioprotective effects of BNP were attributed to the inhibition of cardiomyocyte apoptosis, myocardial fibrosis, cardiac hypertrophy and the AGE–receptor for AGE (RAGE) system as well as normalisation of cardiac AMP-activated protein kinase and endothelial nitric oxide synthase activities.
Conclusions/interpretation
Our results indicate that chronic BNP treatment at low dose improves the metabolic profile and prevents the development of myocardial dysfunction in db/db mice.







Similar content being viewed by others
Abbreviations
- AMPK:
-
AMP-activated protein kinase
- ANP:
-
Atrial natriuretic peptide
- BAX:
-
BCL2-associated X
- BCL:
-
B cell lymphoma
- BNP:
-
Brain natriuretic peptide
- CASP3:
-
Caspase-3
- CVF:
-
Collagen volume fraction
- ITT:
-
Insulin tolerance test
- LV:
-
Left ventricle
- eNOS:
-
Endothelial nitric oxide synthase
- NP:
-
Natriuretic peptide
- NPR:
-
Natriuretic peptide receptor
- RAGE:
-
Receptor for AGE
- UCP1:
-
Uncoupling protein-1
- veh:
-
Vehicle
References
Cheng S, Fox CS, Larson MG et al (2011) Relation of visceral adiposity to circulating natriuretic peptides in ambulatory individuals. Am J Cardiol 108:979–984
Wang TJ, Larson MG, Keyes MJ, Levy D, Benjamin EJ, Vasan RS (2007) Association of plasma natriuretic peptide levels with metabolic risk factors in ambulatory individuals. Circulation 115:1345–1353
Wang TJ, Larson MG, Levy D et al (2004) Impact of obesity on plasma natriuretic peptide levels. Circulation 109:594–600
Khan AM, Cheng S, Magnusson M et al (2011) Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab 96:3242–3249
Bartels ED, Nielsen JM, Bisgaard LS, Goetze JP, Nielsen LB (2010) Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology 151:5218–5225
Gutkowska J, Broderick TL, Bogdan D, Wang D, Lavoie JM, Jankowski M (2009) Downregulation of oxytocin and natriuretic peptides in diabetes: possible implications in cardiomyopathy. J Physiol 587:4725–4736
Mandavia CH, Aroor AR, Demarco VG, Sowers JR (2013) Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci 92:601–608
Oliver PM, Fox JE, Kim R et al (1997) Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci U S A 94:14730–14735
Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS (1998) Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Investig 101:812–818
De Vito P, Di Nardo P, Palmery M, Peluso I, Luly P, Baldini PM (2003) Oxidant-induced pHi/Ca2+ changes in rat aortic smooth muscle cells. The role of atrial natriuretic peptide. Mol Cell Biochem 252:353–362
Kiemer AK, Vollmar AM (2001) The atrial natriuretic peptide regulates the production of inflammatory mediators in macrophages. Ann Rheum Dis 60(Suppl 3):iii68–iii70
Stadler K (2012) Oxidative stress in diabetes. Adv Exp Med Biol 771:272–287
Pfister R, Sharp S, Luben R et al (2011) Mendelian randomization study of B-type natriuretic peptide and type 2 diabetes: evidence of causal association from population studies. PLoS Med 8:e1001112
Miyashita K, Itoh H, Tsujimoto H et al (2009) Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 58:2880–2892
Heinisch BB, Vila G, Resl M et al (2012) B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia 55:1400–1405
Bordicchia M, Liu D, Amri EZ et al (2012) Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Investig 122:1022–1036
Nishikimi T, Maeda N, Matsuoka H (2006) The role of natriuretic peptides in cardioprotection. Cardiovasc Res 69:318–328
Potter LR (2011) Regulation and therapeutic targeting of peptide-activated receptor guanylyl cyclases. Pharmacol Ther 130:71–82
Cheitlin MD, Armstrong WF, Aurigemma GP et al (2003) ACC/AHA/ASE 2003 Guideline Update for the Clinical Application of Echocardiography: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr 16:1091–1110
Poornima IG, Parikh P, Shannon RP (2006) Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res 98:596–605
Cannon B, Nedergaard J (2004) Brown adipose tissue: function and physiological significance. Physiol Rev 84:277–359
Goldin A, Beckman JA, Schmidt AM, Creager MA (2006) Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114:597–605
Meirhaeghe A, Sandhu MS, McCarthy MI et al (2007) Association between the T-381C polymorphism of the brain natriuretic peptide gene and risk of type 2 diabetes in human populations. Hum Mol Genet 16:1343–1350
Olsen MH, Hansen TW, Christensen MK et al (2005) N-terminal pro brain natriuretic peptide is inversely related to metabolic cardiovascular risk factors and the metabolic syndrome. Hypertension 46:660–666
Kim HN, Januzzi JL Jr (2011) Natriuretic peptide testing in heart failure. Circulation 123:2015–2019
Thireau J, Karam S, Fauconnier J et al (2012) Functional evidence for an active role of B-type natriuretic peptide in cardiac remodelling and pro-arrhythmogenicity. Cardiovasc Res 95:59–68
O'Connor CM, Starling RC, Hernandez AF et al (2011) Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 365:32–43
Lenski M, Kazakov A, Marx N, Bohm M, Laufs U (2011) Effects of DPP-4 inhibition on cardiac metabolism and function in mice. J Mol Cell Cardiol 51:906–918
Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, de Meester I (2006) Dipeptidyl-peptidase IV converts intact B-type natriuretic peptide into its des-SerPro form. Clin Chem 52:82–87
Nishikimi T, Kuwahara K, Nakagawa Y, Kangawa K, Minamino N, Nakao K (2013) Complexity of molecular forms of B-type natriuretic peptide in heart failure. Heart 99:677–679
Gower WR Jr, Salhab KF, Foulis WL et al (2000) Regulation of atrial natriuretic peptide gene expression in gastric antrum by fasting. Am J Physiol Regul Integr Comp Physiol 278:R770–R780
Engeli S, Birkenfeld AL, Badin PM et al (2012) Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest 122:4675–4679
Welsh P, McMurray JJ (2012) B-type natriuretic peptide and glycaemia: an emerging cardiometabolic pathway? Diabetologia 55:1240–1243
Marfella R, Di Filippo C, Portoghese M et al (2009) Myocardial lipid accumulation in patients with pressure-overloaded heart and metabolic syndrome. J Lipid Res 50:2314–2323
Ardehali H, Sabbah HN, Burke MA et al (2012) Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail 14:120–129
Khairallah RJ, Khairallah M, Gelinas R et al (2008) Cyclic GMP signaling in cardiomyocytes modulates fatty acid trafficking and prevents triglyceride accumulation. J Mol Cell Cardiol 45:230–239
Bergandi L, Silvagno F, Russo I et al (2003) Insulin stimulates glucose transport via nitric oxide/cyclic GMP pathway in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 23:2215–2221
Nielsen JM, Kristiansen SB, Norregaard R et al (2009) Blockage of receptor for advanced glycation end products prevents development of cardiac dysfunction in db/db type 2 diabetic mice. Eur J Heart Fail 11:638–647
Acknowledgements
We are very grateful to J.-L. Chiasson (Department of Medicine, Chief, Endocrinology Division, CHUM, University of Montreal, QC, Canada) for his valuable comments and criticism.
Funding
This study was supported by grants from the CIHR and Canadian Heart and Stroke Foundation (to JG, MJ) and the Diabetes Action Research and Education Foundation (to TLB). EP was supported by a fellowship grant from the CIHR and Canadian Heart and Stroke Foundation.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
EP had full access to all the data in the study and takes responsibility for the accuracy of the data analysis. JG and MJ are the principal investigators for the project. EP, AM and BAD acquired the data. EP, MJ, TLB and JG conceived and designed the study question. EP and AM analysed the data, and EP, MJ, TLB and JG interpreted the data. EP and MJ drafted the manuscript, and all authors critically revised the manuscript for important intellectual content and have approved the final version.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Methods and Results
(PDF 188 kb)
ESM Table 1
(PDF 126 kb)
ESM Table 2
(PDF 153 kb)
Rights and permissions
About this article
Cite this article
Plante, E., Menaouar, A., Danalache, B.A. et al. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia 57, 1257–1267 (2014). https://doi.org/10.1007/s00125-014-3201-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3201-4