Abstract
Aims/hypothesis
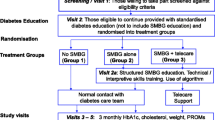
We evaluated whether self-monitoring of blood glucose (SMBG) leads to better glycaemic control (HbA1c) in patients with type 2 diabetes on conventional insulin regimens.
Methods
Patients with type 2 diabetes on a conventional insulin regimen (basal or premixed insulin with or without additional oral glucose-lowering agents) were recruited at study centres led by members of the German Diabetes Association. In a randomised, prospective, open 2 × 2 factorial design, the once-weekly performance of four-point glucose profiles (SMBG +; n = 151 patients) was compared with no SMBG (SMBG −; n = 149), and the measuring and transmitting of HbA1c results to the study centres (HbA1c +; n = 158, of these 82 SMBG − and 76 SMBG +) was compared with HbA1c measurement without disclosure of results (HbA1c −; n = 142, of these 67 SMBG − and 75 SMBG +). Randomised allocation was carried out by a central office, using sequentially numbered, sealed envelopes. The primary endpoint was the reduction of HbA1c compared with baseline after 12 months. Secondary analyses were of therapy intensification in response to higher blood or urinary glucose or HbA1c. Participants and caregivers were not blinded as to the allocation of interventions, whereas the laboratory determining HbA1c remained blinded.
Results
Patient characteristics were balanced across groups. A total of 56 patients dropped out. In completers, HbA1c was reduced in the SMBG + group from 7.3% to 7.0%, i.e. by 0.3% (0.1%, 0.5%) vs SMBG − from 7.3% to 7.0% and 0.3% (0.2%, 0.5%), respectively, the difference being 0.0% (−0.2%, 0.2%) (p = 0.93). The disclosure of HbA1c results had no significant influence, with a difference of 0.1% (−0.1%, 0.4%) (p = 0.28). Values above are mean (95% CI). The ORs for therapy intensification significantly rose as the following increased: proportions of urine samples testing positive for glucose, HbA1c concentrations, and fasting or postprandial glucose concentrations. No important adverse events were associated with the interventions.
Conclusions/interpretation
SMBG profiles once weekly or the disclosure of HbA1c results did not improve glycaemic control in patients with type 2 diabetes on conventional insulin treatment, although indicators of hyperglycaemia increased the likelihood of therapy intensification. Greater intensification may be necessary to impact on glycaemic control.
Trial registration:
www.clinicaltrials.gov (registration code NCT00688363)
Funding:
Deutsche Diabetes-Gesellschaft, Deutsche Diabetes-Stiftung, Bayer Vital GmbH



Similar content being viewed by others
Abbreviations
- LOCF:
-
Last observation carried forward
- SMBG:
-
Self-monitoring of blood glucose
- SMUG:
-
Self-monitoring of urinary glucose
References
DCCT Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes. N Engl J Med 329:977–986
Pickup JC, Kidd J, Burmiston S, Yemane N (2006) Determinants of glycaemic control in type 1 diabetes during intensified therapy with multiple daily insulin injections or continuous subcutaneous insulin infusion: importance of blood glucose variability. Diabetes Metab Res Rev 22:232–237
Raskin P, Bode BW, Marks JB et al (2003) Continuous subcutaneous insulin infusion and multiple daily injection therapy are equally effective in type 2 diabetes: a randomized, parallel-group, 24-week study. Diabetes Care 26:2598–2603
Grüsser M, Hartmann P, Schlottmann N, Joergens V (1996) Structured treatment and teaching programme for type 2 diabetic patients on conventional insulin treatment: evaluation of reimbursement policy. Patient Educ Couns 29:123–130
Bundesärztekammer (BÄK) KBK (2013) Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) (2012) Nationale Versorgungs-Leitlinie Diabetes. Strukturierte Schulungsprogramme – Langfassung. Ärztliches Zentrum für Qualität in der Medizin, Berlin, Germany [in German]; available from www.versorgungsleitlinien.de/themen/diabetes2/dm2_schulung/index_html (accessed 6 January 2014)
Nauck MA, El-Ouaghlidi A, Vardarli I (2009) Blutzuckerselbstkontrolle bei Diabetes mellitus: Plädoyer für ein individuelles Selbstkontrollkonzept/Self-monitoring of blood glucose in diabetes mellitus: arguments for an individualized approach. Dtsch Ärzteblatt Int 106:587–594 [article in German]
Schwedes U, Siebolds M, Mertes G (2002) Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes Care 25:1928–1932
Guerci B, Drouin P, Grange V et al (2003) Self-monitoring of blood glucose significantly improves metabolic control in patients with type 2 diabetes mellitus: the Auto-Surveillance Intervention Active (ASIA) study. Diabetes Metab 29:587–594
Barnett AH, Krentz AJ, Strojek K et al (2008) The efficacy of self-monitoring of blood glucose in the management of patients with type 2 diabetes treated with a gliclazide modified release-based regimen. A multicentre, randomized, parallel-group, 6-month evaluation (DINAMIC 1 study). Diabetes Obes Metab 10:1239–1247
Murata GH, Duckworth WC, Shah JH, Wendel CS, Mohler MJ, Hoffman RM (2009) Blood glucose monitoring is associated with better glycemic control in type 2 diabetes: a database study. J Gen Intern Med 24:48–52
Chen HS, Wu TE, Jap TS, Lin SH, Hsiao LC, Lin HD (2008) Improvement of glycaemia control in subjects with type 2 diabetes by self-monitoring of blood glucose: comparison of two management programs adjusting bedtime insulin dosage. Diabetes Obes Metab 10:34–40
Murata GH, Shah JH, Hoffman RM et al (2003) Intensified blood glucose monitoring improves glycemic control in stable, insulin-treated veterans with type 2 diabetes: the Diabetes Outcomes in Veterans Study (DOVES). Diabetes Care 26:1759–1763
Martin S, Schneider B, Heinemann L et al (2006) Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: an epidemiological cohort study. Diabetologia 49:271–278
Franciosi M, Pellegrini F, de Berardis G et al (2005) Self-monitoring of blood glucose in non-insulin-treated diabetic patients: a longitudinal evaluation of its impact on metabolic control. Diabet Med J Br Diabet Assoc 22:900–906
O'Kane MJ, Bunting B, Copeland M, Coates VE (2008) Efficacy of self monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. Brit Med J 336:1174–1177
Farmer A, Wade A, Goyder E et al (2007) Impact of self monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. Brit Med J 335:132
Welschen LM, Bloemendal E, Nijpels G et al (2005) Self-monitoring of blood glucose in patients with type 2 diabetes who are not using insulin: a systematic review. Diabetes Care 28:1510–1517
Poolsup N, Suksomboon N, Jiamsathit W (2008) Systematic review of the benefits of self-monitoring of blood glucose on glycemic control in type 2 diabetes patients. Diabetes Technol Ther 10(Suppl 1):S51–S66
Towfigh A, Romanova M, Weinreb JE et al (2008) Self-monitoring of blood glucose levels in patients with type 2 diabetes mellitus not taking insulin: a meta-analysis. Am J Manage Care 14:468–475
Polonsky WH, Fisher L, Schikman CH et al (2011) Structured self-monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin-treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care 34:262–267
Farmer AJ, Perera R, Ward A et al (2012) Meta-analysis of individual patient data in randomised trials of self monitoring of blood glucose in people with non-insulin treated type 2 diabetes. BMJ 344:e486
American Diabetes Association (2005) Clinical practice recommendations 2005. Diabetes Care 28(Suppl 1):S1–S79
European Diabetes Policy Group (1999) A desktop guide to type 2 diabetes mellitus. Diabet Med J Br Diabet Assoc 16:716–730
Larsen ML, Hørder M, Mogensen EF (1990) Effect of long-term monitoring of glycosylated hemoglobin levels in insulin-dependent diabetes mellitus. N Engl J Med 323:1021–1025
Nauck MA, Heinemann L, Haastert B et al (2009) A randomized, controlled trial of blood glucose self-monitoring in type 2-diabetic patients receiving conventional insulin treatment (abstract). Diabetologia 52(Suppl 1):S41
Matthaei S, Bierwirth R, Fritsche A, et al. (2008) Medikamentöse antihyperglykämische Therapie des Diabetes mellitus Typ 2. In: Scherbaum WA, Haak T (eds) Evidenzbasierte Leitlinie der Deutschen Diabetes-Gesellschaft, available from www.arztbibliothek.de/mdb/downloads/ddiabetesg/antihyperglykaemische-therapie-lang.pdf [in German] (accessed 6 January, 2014)
Brown H, Prescott R (2006) Applied mixed models in medicine. Wiley, Bognor Regis
Starostina EG, Antsiferov M, Galstyan GR et al (1994) Effectiveness and cost-benefit analysis of intensive treatment and teaching programmes for type 1 (insulin-dependent) diabetes mellitus in Moscow—blood glucose versus urine glucose self-monitoring. Diabetologia 37:170–176
Riddle MC, Rosenstock J, Gerich J (2003) The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26:3080–3086
Yki-Järvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M (1999) Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. A randomized, controlled trial. Ann Intern Med 130:389–396
Yki-Järvinen H, Kauppinen-Makelin R, Tiikkainen M et al (2006) Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 49:442–451
Holman RR, Thorne KI, Farmer AJ et al (2007) Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med 357:1716–1730
Janka HU, Plewe G, Riddle MC, Kliebe-Frisch C, Schweitzer MA, Yki-Järvinen H (2005) Comparison of basal insulin added to oral agents versus twice-daily premixed insulin as initial insulin therapy for type 2 diabetes. Diabetes Care 28:254–259
Stone MA, Wilkinson JC, Charpentier G et al (2010) Evaluation and comparison of guidelines for the management of people with type 2 diabetes from eight European countries. Diabetes Res Clin Pract 87:252–260
Boussageon R, Bejan-Angoulvant T, Saadatian-Elahi M et al (2011) Effect of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in type 2 diabetes: meta-analysis of randomised controlled trials. Brit Med J 343:d4169
Beluchin E, Baz L, Müller N et al (2013) Frequency of self-adjustment of insulin dose and metabolic control in type 2 diabetes—is there an association? Diabet Med J Br Diabet Assoc 30:e91–e94
Turchin A, Shubina M, Chodos AH, Einbinder JS, Pendergrass ML (2008) Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation 117:623–628
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Turner RC, Cull CA, Frighi V, Holman RR (1999) Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. J Am Med Assoc 281:2005–2012
Scherbaum WA, Ohmann C, Abholz HH, Dragano N, Lankisch M (2008) Effect of the frequency of self-monitoring blood glucose in patients with type 2 diabetes treated with oral antidiabetic drugs—a multi-centre, randomized controlled trial. PLoS ONE 3:e3087
Acknowledgements
The study physician M. Gutzeit and the study nurse S. Ciossek, both Diabeteszentrum Bad Lauterberg, Bad Lauterberg im Harz, Germany, helped compile and analyse data. We also want to thank the study teams from the sites listed in the Appendix.
Funding
We are indebted to the Deutsche Diabetes-Gesellschaft for supporting this study and for providing logistic assistance through the Clinical Trials Study Group (Kommission Klinische Studien der DDG). We also thank Bayer Vital, Diabetes Care, Cologne, Germany and the Deutsche Diabetes-Stiftung, Munich, Germany for additional funding. The funders had no influence on the study protocol, data analysis, or the presentation and interpretation of results, which were entirely the responsibility of the study group represented by the authors.
Duality of interest
MiAN has received research grants (to his institution, the Diabeteszentrum Bad Lauterberg) from: AstraZeneca; Bayer Vital; Berlin-Chemie/Menarini; Bionime; Boehringer Ingelheim; Eli Lilly; Merck; Sharp & Dohme MetaCure; Novartis Pharma; Novo Nordisk Pharma; Roche Pharma; and Tolerx. He has also received consulting fees and/or honoraria for membership of advisory boards and for speaking from Amylin Pharmaceuticals, AstraZeneca, Berlin-Chemie/Menarini, Boehringer Ingelheim, Bristol–Myers Squibb, Diartis Pharmaceuticals, Eli Lilly, F. Hoffmann-LaRoche, GlaxoSmithKline, Intarcia Therapeutics Lifescan, MannKind Corporation, Merck Sharp & Dohme, Novartis Pharma, NovoNordisk, Sanofi-Aventis Pharma, Takeda, Versartis and Wyeth Research. These fees/honoraria included reimbursement for travel expenses in connection with the above-mentioned activities. MiAN owns no stock and is employed by Diabeteszentrum Bad Lauterberg, Germany.
BH has no duality of interest in relation to this manuscript.
CT is employed by the Ostfalia University of Applied Sciences. He has received consulting fees and/or honoraria for speaking from AstraZeneca, Novo Nordisk Pharma, German sickness funds and the National Association of Statutory Health Insurance Physicians (KBV).
UAM received a research grant from Roche Diagnostics, Germany and institutional education grants from Novo Nordisk and Merck Sharp & Dohme.
MaAN has received research grants (to his institution, Universitätsmedizin Greifswald) from Becton Dickinson, Bio-Rad, Radiometer, Roche and Siemens Healthcare Diagnostics. He has received consulting fees and/or honoraria for membership of advisory boards and for speaking from Becton Dickinson, Bio-Rad, Roche and Siemens Healthcare Diagnostics. These fees/honoraria included reimbursement for travel expenses in connection with the above-mentioned activities. Matthias AN owns no stock and is employed by Universitätsmedizin Greifswald, Germany.
LH, in his position as CEO of Profil when this study was performed, has received grants from a number of companies for performing clinical trials related to SMBG and continuous glucose monitoring. He has also received honoraria from many of these companies for consultancy and speaking activities.
Contribution statement
MiAN, BH, CT, MaAN and LH served on the Steering Committee designing, performing and analysing the study. They wrote the manuscript and collectively decided to publish the manuscript. BH performed the statistical analysis. UAM contributed to the analysis and interpretation of the data. All authors participated in the drafting of the article or revising it critically for important intellectual content, and have given final approval of the version to be submitted for publication.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
Additional members of the Clinical Trials Study Group are listed in the Appendix.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig. 1
(PDF 10 kb)
ESM Fig. 2
(PDF 10 kb)
Appendix
Appendix
In addition to the persons listed as authors, the following are also members of the Clinical Trials Study Group of the German Diabetes Association: P. Sawicki, Cologne; B. Böhm, Ulm; E. Rehring, Bad Lauterberg im Harz; H.-G. Ley, Marl; B. Gallwitz, Tübingen; S. Lange, Cologne; A. Tytko, Bad Lauterberg; R. Lundershausen, Erfurt; S. Martin, Düsseldorf; M. Gutzeit, Bad Lauterberg; Ch. von Boxberg, Leverkusen; M. Füchtenbusch, Munich. We acknowledge the following individuals for taking care of the patients studied at the associated study centres: R. Naumann, Schöppenstedt; R. Bellmann, Berlin; M. Jäger, Höchst (Breuberg/Odenwald); M. Gutzeit and A. Hinz, Bad Lauterberg; A. Eidner, Jena; A. Kamke and R. Radke, Burg/Spreewald; Z. Kourbanova, Langenfeld; U. Weller, Dorsten; M. Füchtenbusch, Munich; A. Schmidt-Reinwald, Waldrach; S. Maxeiner, Bosenheim; A. Hendel, Grassau; R. Böhme, Nordhausen; Th. Behnke, Neuwied; P. Krege, Emsdetten; U. Schmitz, Krefeld; A.-W. Bödecker, Wiehl; A. Rieth-Kunert, Stade; Stefan Fels, Oldenburg; W. Neumann, Selters/Westerwald; G. Willms, Leverkusen; H.-G. Ley, Marl; C. Jödicke, Bad Lauterberg; P. Koch, Bad Harzburg; Ch. Mulch-Wiemer, Bad Nauheim; M. Pfeiffer, Gronau; F. Fueting, Nassau; V. J. Jung, Waldkraiburg; J. Grossmann, Mönchengladbach; R. Hildebrandt, Clausthal-Zellerfeld; E.-M. Oerter, Würzburg; U. Preuß, Datteln; M. Friedrichs, Bad Lauterberg; M. Leupold, Borna; S. Gölz, Esslingen; H. Fischer, Düren; U. Warmers, Bitburg; D. Schoch, Berlin; K. Nowack, Torgau; J. Lemmerhirt, Cuxhaven; F. Klein, Schenklengsfeld; I. Niemetz, Kassel; K. Wollersen, Freiburg. All centres listed are in Germany.
Rights and permissions
About this article
Cite this article
Nauck, M.A., Haastert, B., Trautner, C. et al. A randomised, controlled trial of self-monitoring of blood glucose in patients with type 2 diabetes receiving conventional insulin treatment. Diabetologia 57, 868–877 (2014). https://doi.org/10.1007/s00125-014-3168-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3168-1