Abstract
Aims/hypothesis
The aim of this meta-analysis is to determine the predictive value of diabetic retinopathy in differentiating diabetic nephropathy from non-diabetic renal diseases in patients with type 2 diabetes and renal disease.
Methods
Medline and Embase databases were searched from inception to February 2012. Renal biopsy studies of participants with type 2 diabetes were included if they contained data with measurements of diabetic retinopathy. Pooled sensitivity, specificity, positive predictive value, negative predictive value and other diagnostic indices were evaluated using a random-effects model.
Results
The meta-analysis investigated 26 papers with 2012 patients. The pooled sensitivity and specificity of diabetic retinopathy to predict diabetic nephropathy were 0.65 (95% CI 0.62, 0.68) and 0.75 (95% CI 0.73, 0.78), respectively. The pooled positive and negative predictive value of diabetic retinopathy to predict diabetic nephropathy were 0.72 (95% CI 0.68, 0.75) and 0.69 (95% CI 0.67, 0.72), respectively. The area under the summary receiver operating characteristic curve was 0.75, and the diagnostic odds ratio was 5.67 (95% CI 3.45, 9.34). For proliferative diabetic retinopathy, the pooled sensitivity was 0.25 (95% CI 0.16, 0.35), while the specificity was 0.98 (95% CI 0.92, 1.00). There was heterogeneity among studies (p < 0.001), and no publishing bias was identified.
Conclusions/interpretation
Diabetic retinopathy is useful in diagnosing or screening for diabetic nephropathy in patients with type 2 diabetes and renal disease. Proliferative diabetic retinopathy may be a highly specific indicator for diabetic nephropathy.
Similar content being viewed by others
Introduction
The incidence and prevalence of diabetes mellitus have been increasing. New figures indicate that, if no urgent action is taken, the number of people living with diabetes will rise from 366 million in 2011 to 552 million by 2030 [1]. In adults, type 2 diabetes mellitus accounts for 90–95% of all diagnosed cases of diabetes in the USA [2]. Approximately 40% of people with diabetes develop diabetic nephropathy (DN), which has become the leading cause of end-stage renal disease (ESRD) in developed countries [3]. From United States Renal Data System reports, the adjusted rate of prevalent ESRD due to diabetes rose 2.2% to 647 per million people in 2009, and the total medical care expenditure for ESRD rose 3.1%, reaching US$29 billion dollars [4].
Kidney biopsy can discriminate DN from non-diabetic renal disease (NDRD), but it is invasive and not suitable for every patient. NDRD is rare in type 1 diabetes mellitus, particularly in patients with a history of diabetes of >10 years [5]; however, reports of the prevalence of NDRD in type 2 diabetes mellitus have varied widely from 10% to 85% [6–10]. One joint analysis of available data on the prevalence of NDRD among patients with type 2 diabetes mellitus revealed that NDRD was evident on kidney biopsy in ∼22% of European and 26.7% of Asian patients [11]. Furthermore, the treatment and prognosis of DN and NDRD are different. Research has shown that diabetic patients with NDRD have significantly better renal outcomes than patients with biopsy-proven DN, since many NDRDs are treatable, and even remittable [12].
Assessment of diabetic retinopathy (DR) is inexpensive and could be routinely performed during outpatient screening for chronic complications of diabetes. Indeed, previous literature has shown that DR may be helpful in distinguishing the type of kidney pathology in patients with type 2 diabetes mellitus and renal disease [13–15]. However, the results of these studies are diverse and have been found to have variable predictive value in the different series. In addition, most of the available data are from retrospective studies with small samples that lack a quantified standard. Therefore, it is worth comprehensively reviewing the data on the predictive role of DR in biopsy studies. This meta-analysis focused on both prospective and retrospective biopsy studies, and aims to estimate the overall capacity of DR for predicting DN in type 2 patients with diabetes mellitus and renal disease.
Methods
Search strategy
The databases searched included Medline and Embase, from the time of their inception to February 2012. The medical subject headings (MeSH) were ‘Biopsy’ or ‘Pathology’ and ‘Diabetic nephropathy/diagnosis/aetiology/pathology’. The references from retrieved articles and reviews identified in the search were manually inspected to verify further articles. One reviewer (F.H.) performed the search, while a second (X.X.) confirmed the process.
Study selection
The search yielded 3,361 articles, which were assessed using titles, abstracts and/or full articles. Only papers published in English were included. The inclusion criteria were: (1) patients with type 2 diabetes mellitus and renal disease; (2) identification of renal diseases based on kidney biopsy findings; (3) presence of DR and numbers of patients classified in each renal disease group. We included the latest publication when more than one paper was published on a study. While screening the citations, two reviewers (F.H. and X.X.) independently reviewed the search results to determine article inclusion. In cases of discord, a consensus was reached through discussion with the senior author (F.X.H.).
Data extraction and quality assessment
The same investigators (F.H. and X.X.) each retrieved data using standardised forms, obtaining information on study design, author, publication year, percentage of men, duration of diabetes, presence of baseline proteinuria, methods of evaluating DR, and the inclusion criteria to select patients. Data were collected at baseline in the case of longitudinal studies. The numbers of true-positive, false-positive, true-negative and false-negative results were calculated for each study. Study quality was assessed with the quality assessment of studies of diagnostic accuracy included in systematic reviews (QUADAS) checklist (maximum score 14) [16]. The checklist is structured as a list of 14 questions that should be answered ‘yes,’ ‘no’ or ‘unclear.’
Statistical analysis
Sensitivity, specificity, positive predictive value, negative predictive value and diagnostic odds ratios (DORs) were calculated for each study after construction of a 2 × 2 table. Cells with a value of ‘0’ in the 2 × 2 tables were replaced with ‘0.5’ for pooling purposes. The pooled estimates with 95% CIs were calculated using a random-effects model [17]. A summary receiver operating characteristic (sROC) curve was performed to assess the interaction between sensitivity and specificity. A weighted AUC was obtained to estimate the diagnostic performance. The I 2 test was used to quantify the degree of heterogeneity among studies, with I 2 values of 25%, 50% and 75% being tentatively considered low, moderate and high heterogeneity, respectively [18]. The potential presence of publication bias was tested for using the Egger test [19].
Analyses were performed using Stata statistical software v.11.0 for Windows (Stata Corp, College Station, TX, USA) and Meta-DiSc software (Madrid, Spain) [20]. Statistical tests were two-sided and used a significance level of p < 0.05.
Results
Literature search results and study characteristics
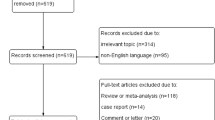
The literature search initially identified 3361 articles, which were reduced to 48 after titles and abstracts had been read. After full-text evaluation, 26 papers remained for analysis [8–10, 12, 14, 15, 21–40], including nine prospective studies and 17 retrospective ones.
The 26 articles identified above involved 2,012 participants for inclusion. The quality of original studies was checked according to QUADAS, and all of the studies had high scores (≥11). Details of the study characteristics and their corresponding QUADAS scores are summarised in Table 1. The proportion of men in the studies ranged between 47% and 94% (weighted average 62%). The mean duration of diabetes in the studies was 5–12 years. In prospective studies, the participants all had proteinuria when the biopsies were performed, which were mostly macroalbuminuria, except for one study, which was limited to microalbuminuria [21]. Different methods were used to assess DR: ophthalmoscopy after mydriasis in four studies [21, 22, 24, 30, 36]; fundus photography after mydriasis in one study [27]; ophthalmoscopy without mydriasis in four studies [15, 23, 26, 28, 29, 33, 34, 40]; and in nine studies no relevant details were provided [8, 10, 25, 31–33, 35, 38, 39]. Of the nine prospective studies assessed, five were screening studies conducted on consecutive patients with type 2 diabetes mellitus and proteinuria [21–25], while the remaining four were conducted on selected type 2 diabetes mellitus populations using criteria for the biopsy for type 1 diabetes mellitus (microhaematuria, absence of DR, atypical change in renal function, or immunological abnormalities) [26–29]. However, in most retrospective studies, renal biopsy indications were based on clinical suspicion of NDRD (microhaematuria, atypical change in renal function, overt proteinuria, without unified and clear criteria).
DR predicting DN
Forest plots of the pooled sensitivity and specificity are shown in Fig. 1. In the five screening prospective studies, the sensitivity ranged from 0.59 to 1.00 (pooled sensitivity 0.70, 95% CI 0.63, 0.76), and the specificity ranged from 0.41 to 1.00 (pooled specificity 0.73, 95% CI 0.65, 0.80). For the remaining four selected samples of the prospective studies, the sensitivity ranged from 0.35 to 0.71 (pooled sensitivity 0.50, 95% CI 0.40, 0.60), and the specificity ranged from 0.27 to 0.88 (pooled specificity 0.77, 95% CI 0.69, 0.84). The overall pooled sensitivity and specificity for all the prospective studies were 0.64 (95% CI 0.58, 0.69) and 0.75 (95% CI 0.70, 0.80), respectively. Finally, taking all 26 prospective and retrospective studies together, the total pooled sensitivity and specificity were 0.65 (95% CI 0.62, 0.68) and 0.75 (95% CI 0.73, 0.78), respectively.
Forest plots of pooled sensitivity (a) and specificity (b) of DR predicting biopsy-proven DN in separate and combined groups. T2DM, type 2 diabetes mellitus. T1DM, type 1 diabetes mellitus. The black circles and horizontal lines represent the study-specific index of diagnosis and corresponding 95% CI, respectively. The diamond and vertical lines/brackets represent the pooled estimate with 95% CI
Forest plots of the pooled DOR are shown in Fig. 2. In the five screening prospective studies, the pooled DOR ranged from 1.00 to 45.84 (pooled DOR 8.98, 95% CI 2.01, 40.19). For the remaining four selected samples of the prospective studies, the pooled DOR ranged from 0.36 to 10.93 (pooled DOR 2.28, 95% CI 0.46, 11.22). The overall pooled DOR for all the prospective studies was 4.65 (95% CI 1.62, 13.35). When all 26 prospective and retrospective studies were taken together, the total pooled DOR was 5.67 (95% CI 3.45, 9.34). The pooled positive predictive value of DR predicting DN for all 26 papers was 0.72 (95% CI 0.68, 0.75) (Fig. 3a), while the pooled negative predictive value was 0.69 (95% CI 0.67, 0.72) (Fig. 3b).
Forest plots of pooled DORs of DR predicting biopsy-proven DN in separate and combined groups. T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus. The black circles and horizontal lines represent the study-specific index of diagnosis and corresponding 95% CI, respectively. The diamond and vertical lines/brackets represent the pooled estimate with 95% CI
Forest plots of pooled positive (a) and negative (b) predictive values of DR predicting biopsy-proven DN in separate and combined groups. T2DM, type 2 diabetes mellitus; T1DM, type 1 diabetes mellitus. The black circles and horizontal lines represent the study-specific index of diagnosis and corresponding 95% CI, respectively. The diamond and vertical lines/brackets represent the pooled estimate with its 95% CI
The sROC curves showing sensitivity vs 1 − specificity from individual studies was not positioned near the desirable upper left corner (Fig. 4). The AUC was 0.82 (SEM 0.08) in the prospective consecutive screening subgroup, 0.73 (SEM 0.08) in nine prospective studies, and 0.75 (SEM 0.03) in all 26 studies.
sROC curves of DR predicting biopsy-proven DN in separate and combined groups. (a) Symmetric sROC curve of the screening subgroup; AUC, 0.8194; SE (AUC), 0.0881; Q*, 0.7530; SE (Q*), 0.0791; (b) sROC curve of the nine prospective studies; AUC, 0.7288; SE (AUC), 0.0797; Q*, 0.6760; SE (Q*), 0.0647 (c) sROC curve of all 26 studies included in the meta-analysis; AUC, 0.7532; SE (AUC), 0.0344; Q*, 0.6961; SE (Q*), 0.0285. Q* (i.e. the Q value) is the maximum joint sensitivity and specificity
The I 2 test detected moderate to high heterogeneity among studies. Therefore, random-effects models were used for the meta-analysis. The Egger test showed no significant publication bias (p = 0.77).
Proliferative DR (PDR) predicting DN
Figure 5 shows the sROC curves and the forest plots of the pooled sensitivity, specificity, positive predictive value and negative value of PDR predicting biopsy-proven DN. Only four studies evaluated DR graded as simple or proliferative DR [8, 21, 24, 28]. The sensitivity ranged from 0.03 to 0.57 (pooled sensitivity 0.25, 95% CI 0.16, 0.35), while the specificity ranged from 0.93 to 1.00 (pooled specificity 0.98, 95% CI 0.92, 1.00). The pooled positive predictive value was 0.96 (95% CI 0.79, 1.00), while the pooled negative predictive value was 0.48 (95% CI 0.39, 0.57). The sROC curves showing sensitivity vs 1 − specificity from individual studies was positioned near the desirable upper left corner with an AUC of 0.99 (SEM 0.05). The I 2 test detected high heterogeneity among studies for sensitivity (I 2 = 85.3%, p < 0.001) and negative predictive value (I 2 = 93.2%, p < 0.001), but heterogeneity was low for specificity (I 2 = 0.5%, p = 0.389) and positive predictive value (I 2 = 45.9%, p = 0.136).
Forest plots of (a) pooled sensitivity, specificity, positive predictive value, negative predictive value. The black circles and horizontal lines represent the study-specific index of diagnosis and corresponding 95% CI, respectively. The diamond and vertical lines/brackets represent the pooled estimate with its 95% CI. (b) sROC curve of proliferative DR predicting biopsy-proven diabetic nephropathy; AUC, 0.9887; SE (AUC), 0.0459; Q*, 0.9508; SE (Q*), 0.1008. Q* (i.e. the Q value) is the maximum joint sensitivity and specificity
Discussion
This meta-analysis shows that the pooled sensitivity and specificity for the presence of DR differentiating DN from NDRD among patients with type 2 diabetes mellitus and renal diseases were 0.65 and 0.75, respectively. Meanwhile, the pooled positive and negative predictive values were 0.72 and 0.69. In addition, DOR was 5.67 and AUC was 0.75. With regard to the subgroup analysis, DR had a higher predictive value in the screening studies than in those with selected patients based on biopsy criteria for type 1 diabetes mellitus. Moreover, predictive results of DR between prospective and retrospective studies were not very different. Our data also show that PDR (an advanced stage of DR) had a high pooled specificity (0.98) and high pooled positive predictive value (0.96), although the pooled sensitivity and negative predictive value were 0.25 and 0.48, respectively. Furthermore, the AUC of 0.99 represented good discrimination.
The strength of this analysis is that it is the first meta-analysis combining data from previous prospective and retrospective biopsy studies on the predictive accuracy of DR for the clinical differentiation of DN. Guidelines for diabetes and chronic kidney disease (CKD) summarised the predictive value of DR for diagnosis of diabetic kidney disease in biopsy studies until 2005 [3]. Estimates of renal disease in diabetic patients are defined by functional abnormalities, such as microalbuminuria. However, it is important to exclude NDRD because some cases require targeted therapy. Kidney biopsy is the gold standard method for identifying DN, but it cannot be performed on all patients because of contraindications. Furthermore, at least 24 h of observation are recommended after a percutaneous kidney biopsy to assess potential complications [41]. In contrast, assessment of DR is very convenient and is routinely performed as part of a physical examination in outpatient departments. DR and DN are both microvascular complications of diabetes, and some authors have identified correlations of anatomical measures between them. Retinopathy severity was found to be associated with renal anatomical measures when other risk factors were controlled for in patients with type 1 diabetes mellitus [42]. Although DR was found to be an important predictor, it is not known whether its presence can completely differentiate DN from NDRD. Along these lines, our previous study showed that the absence of DR together with a short duration of diabetes may be a useful indication for renal biopsy in patients with type 2 diabetes mellitus and overt proteinuria [34]. One study suggested that diabetes mellitus duration of >10 years together with retinopathy did not exclude NDRD in patients with type 2 diabetes mellitus [37]. A differential diagnostic model composed of five clinical indices (diabetes duration, systolic blood pressure, HbA1c, haematuria and DR) had an advantage in the clinical prediction of DN, with a sensitivity of 90.0% and a specificity of 92.0% [25]. The present studies indicated that DR alone had an imperfect predictive value, and that perhaps more clinical features should be confirmed to construct more precise diagnostic criteria for making a distinction between DN and NDRD.
The 26 studies identified for our meta-analysis varied in certain characteristics. For instance, studies included patients with microalbuminuria (20–200 mg/day) [21], macroalbuminuria with ranges defined as from >300 mg/day to >3 g/day [9, 14, 15, 22–29, 31, 34, 36–39], or not defined clearly. In addition, there was significant diversity in the methods used to assess DR, and only seven studies applied gold standards for DR screening [21, 22, 24, 27, 30, 36, 37, 43]. A third issue was the different categories of renal pathology between studies. Most studies divided patients into two groups (DN group and NDRD group). Otherwise, a near-normal renal structure was taken as NDRD in one study for analysis [21], and one study omitted a small proportion of overlapping cases (1.8%) and an ambiguous case [25], whereas in the other studies these overlapping cases were classified in the NDRD groups.
We also found significant heterogeneity, which may be explained by the following limitations. First, only articles published in English were included, although the Egger test did not indicate publication bias. Second, baseline risk factors were not standardised between studies. Of these, ethnic origin affected the susceptibility to DR development even after adjustment for other risk factors [44, 45], although DR showed no significant difference in diagnostic accuracy among the Asian, European and African-American populations (data not shown) in our analysis. The duration of diabetes, a strong risk factor for the development of DR, varied widely from 5 to 12 years in all the studies [46]. Moreover, hyperglycaemia, hypertension and dyslipidaemia have all been confirmed to have an effect on DR [47–49]. However, individual patient data on these risk factors were not available to allow us to explore the heterogeneity in more detail. Third, our results showed that PDR was a high specific indicator for the diagnosis of DN. However, the findings should be interpreted cautiously because they were based on a small sample (169 patients).
In conclusion, current evidence suggests a potential role for DR in predicting DN in type 2 diabetes mellitus with renal disease. Although the overall test performance was not as high as expected, measuring DR may be considered useful for predicting DN in the light of its simplicity and non-invasiveness.
Abbreviations
- DN:
-
Diabetic nephropathy
- DOR:
-
Diagnostic odds ratio
- DR:
-
Diabetic retinopathy
- ESRD:
-
End-stage renal disease
- NDRD:
-
Non-diabetic renal disease
- PDR:
-
Proliferative diabetic retinopathy
- QUADAS:
-
Quality assessment of studies of diagnostic accuracy included in systematic reviews
- sROC:
-
Summary receiver operating characteristic
References
The International Diabetes Federation (2011) International Diabetes Federation’s 5th edition of the Diabetes atlas, 5th edn. Available from www.idf.org, accessed 10 March 2012
Centers for Disease Control and Prevention (2011) National diabetes fact sheet. Available from www.cdc.gov, accessed 10 March 2012
KDOQI (2007) KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis 49:S12–S154
Renal Data System US (2011) USRDS 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. US Renal Data System, Bethesda
Wirta O, Helin H, Mustonen J, Kuittinen E, Savela T, Pasternack A (2000) Renal findings and glomerular pathology in diabetic subjects. Nephron 84:236–242
Lee EY, Chung CH, Choi SO (1999) Non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Yonsei Med J 40:321–326
Prakash J, Sen D, Usha et al (2001) Non-diabetic renal disease in patients with type 2 diabetes mellitus. J Assoc Phys India 49:415–420
Olsen S, Mogensen CE (1996) How often is NIDDM complicated with non-diabetic renal disease? An analysis of renal biopsies and the literature. Diabetologia 39:1638–1645
Nzerue CM, Hewan-Lowe K, Harvey P et al (2000) Prevalence of non-diabetic renal disease among African-American patients with type II diabetes mellitus. Scand J Urol Nephrol 34:331–335
Chong YB, Keng TC, Tan LP et al (2012) Clinical predictors of non-diabetic renal disease and role of renal biopsy in diabetic patients with renal involvement: a single centre review. Ren Fail 34:323–328
Zukowska-Szczechowska E, Tomaszewski M (2004) Renal affection in patients with diabetes mellitus is not always caused by diabetic nephropathy. Rocz Akad Med Bialymst 49:185–189
Chang TI, Park JT, Kim JK et al (2011) Renal outcomes in patients with type 2 diabetes with or without coexisting non-diabetic renal disease. Diabetes Res Clin Pract 92:198–204
Parving HH, Gall MA, Skott P et al (1992) Prevalence and causes of albuminuria in non-insulin-dependent diabetic patients. Kidney Int 41:758–762
Tone A, Shikata K, Matsuda M et al (2005) Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract 69:237–242
Mou S, Wang Q, Liu J et al (2010) Prevalence of non-diabetic renal disease in patients with type 2 diabetes. Diabetes Res Clin Pract 87:354–359
Whiting PF, Westwood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J (2006) Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 6:9
Der Simonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7:177–188
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327:557–560
Egger M, Davey SG, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple graphical test. BMJ 315:629–634
Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A (2006) Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Meth 6:31
Brocco E, Fioretto P, Mauer M et al (1997) Renal structure and function in non-insulin dependent diabetic patients with microalbuminuria. Kidney Int Suppl 63:S40–S44
Mak SK, Gwi E, Chan KW et al (1997) Clinical predictors of non-diabetic renal disease in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant 12:2588–2591
Christensen PK, Gall MA, Parving HH (2000) Course of glomerular filtration rate in albuminuric type 2 diabetic patients with or without diabetic glomerulopathy. Diabetes Care 23:B14–B20
Suzuki D, Takano H, Toyoda M et al (2001) Evaluation of renal biopsy samples of patients with diabetic nephropathy. Intern Med 40:1077–1084
Zhou J, Chen X, Xie Y, Li J, Yamanaka N, Tong X (2008) A differential diagnostic model of diabetic nephropathy and non-diabetic renal diseases. Nephrol Dial Transplant 23:1940–1945
John GT, Date A, Korula A, Jeyaseelan L, Shastry JC, Jacob CK (1994) Non-diabetic renal disease in noninsulin-dependent diabetics in a south Indian hospital. Nephron 67:441–443
Christensen PK, Larsen S, Horn T, Olsen S, Parving HH (2001) Renal function and structure in albuminuric type 2 diabetic patients without retinopathy. Nephrol Dial Transplant 16:2337–2347
Wong TY, Choi PC, Szeto CC et al (2002) Renal outcome in type 2 diabetic patients with or without coexisting non-diabetic nephropathies. Diabetes Care 25:900–905
Serra A, Romero R, Bayes B, Lopez D, Bonet J (2002) Is there a need for changes in renal biopsy criteria in proteinuria in type 2 diabetes? Diabetes Res Clin Pract 58:149–153
Amoah E, Glickman JL, Malchoff CD, Sturgill BC, Kaiser DL, Bolton WK (1988) Clinical identification of nondiabetic renal disease in diabetic patients with type I and type II disease presenting with renal dysfunction. Am J Nephrol 8:204–211
Kleinknecht D, Bennis D, Altman JJ (1992) Increased prevalence of non-diabetic renal pathology in type II diabetes mellitus. Nephrol Dial Transplant 7:1258–1259
Richards NT, Greaves I, Lee SJ, Howie AJ, Adu D, Michael J (1992) Increased prevalence of renal biopsy findings other than diabetic glomerulopathy in type II diabetes mellitus. Nephrol Dial Transplant 7:397–399
Soni SS, Gowrishankar S, Kishan AG, Raman A (2006) Non-diabetic renal disease in type 2 diabetes mellitus. Nephrology 11:533–537
Huang F, Yang Q, Chen L, Tang S, Liu W, Yu X (2007) Renal pathological change in patients with type 2 diabetes is not always diabetic nephropathy: a report of 52 cases. Clin Nephrol 67:293–297
Pham TT, Sim JJ, Kujubu DA, Liu IL, Kumar VA (2007) Prevalence of nondiabetic renal disease in diabetic patients. Am J Nephrol 27:322–328
Akimoto T, Ito C, Saito O et al (2008) Microscopic hematuria and diabetic glomerulosclerosis: clinicopathological analysis of type 2 diabetic patients associated with overt proteinuria. Nephron Clin Pract 109:c119–c126
Lin YL, Peng SJ, Ferng SH, Tzen CY, Yang CS (2009) Clinical indicators which necessitate renal biopsy in type 2 diabetes mellitus patients with renal disease. Int J Clin Pract 63:1167–1176
Ghani AA, Waheeb SA, Sahow AA, Hussain N (2009) Renal biopsy in patients with type 2 diabetes mellitus: indications and nature of the lesions. Ann Saudi Med 29:450–453
Chawarnkul O, Vareesangthip K, Ongajyooth L, Cheunsuchon B, Parichatikanond P (2009) Non-diabetic glomerular disease in type II DM: 10 years experience. J Med Assoc Thai 92:S57–S60
Bi H, Chen N, Ling G, Yuan S, Huang G, Liu R (2011) Nondiabetic renal disease in type 2 diabetic patients: a review of our experience in 220 cases. Ren Fail 33:26–30
Whittier WL, Korbet SM (2004) Renal biopsy: update. Curr Opin Nephrol Hypertens 13:661–665
Klein R, Zinman B, Gardiner R et al (2005) The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients: the Renin-Angiotensin System Study. Diabetes 54:527–533
National Health and Medical Research Council (2008) Guidelines for the management of diabetic retinopathy. Commonwealth of Australia, Canberra
Wong TY, Klein R, Islam FM et al (2006) Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 141:446–455
Stolk RP, van Schooneveld MJ, Cruickshank JK et al (2008) Retinal vascular lesions in patients of Caucasian and Asian origin with type 2 diabetes: baseline results from the ADVANCE Retinal Measurements (AdRem) study. Diabetes Care 31:708–713
Fong DS, Aiello L, Gardner TW et al (2003) Diabetic retinopathy. Diabetes Care 26:226–229
UK Prospective Diabetes Study (UKPDS) Group (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 12(352):854–865
UK Prospective Diabetes Study Group (1998) Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317:708–713
ACCORD Study Group, ACCORD Eye Study Group, Chew EY et al (2008) Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med 363:233–244
Funding
This work was supported by the grants from the National Key Basic Research Program of China (grant number 2011CB504005), the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number 2011BAI10B05), the National Natural Science Foundation of China (grant number 81170765), and the Guangdong Natural Science Foundation (grant number S2011020002359).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
FH originated the study, acquired data, analysed statistics, interpreted data, drafted the manuscript, and critically revised the manuscript. XX originated the study, acquired data, analysed statistics, interpreted data, drafted the manuscript, and critically revised the manuscript. FXH designed the study, interpreted data and critically revised the manuscript. XFW analysed and interpreted data, and critically revised the manuscript. XQY interpreted data and critically revised the manuscript for important intellectual content. All authors have approved the final version.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. He and X. Xia contributed equally to this study.
Rights and permissions
About this article
Cite this article
He, F., Xia, X., Wu, X.F. et al. Diabetic retinopathy in predicting diabetic nephropathy in patients with type 2 diabetes and renal disease: a meta-analysis. Diabetologia 56, 457–466 (2013). https://doi.org/10.1007/s00125-012-2796-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-012-2796-6