Abstract
Aims/hypothesis
Glucose-stimulated insulin secretion is dependent on the electrical activity of beta cells; hence, genes encoding beta cell ion channels are potential candidate genes for type 2 diabetes. The gene encoding the voltage-dependent Ca2+ channel CaV2.3 (CACNA1E), telomeric to a region that has shown suggestive linkage to type 2 diabetes (1q21-q25), has been ascribed a role for second-phase insulin secretion.
Methods
Based upon the genotyping of 52 haplotype tagging single nucleotide polymorphisms (SNPs) in a type 2 diabetes case–control sample (n = 1,467), we selected five SNPs that were nominally associated with type 2 diabetes and genotyped them in the following groups (1) a new case–control sample of 6,570 individuals from Sweden; (2) 2,293 individuals from the Botnia prospective cohort; and (3) 935 individuals with insulin secretion data from an IVGTT.
Results
The rs679931 TT genotype was associated with (1) an increased risk of type 2 diabetes in the Botnia case–control sample [odds ratio (OR) 1.4, 95% CI 1.0–2.0, p = 0.06] and in the replication sample (OR 1.2, 95% CI 1.0–1.5, p = 0.01 one-tailed), with a combined OR of 1.3 (95% CI 1.1–1.5, p = 0.004 two-tailed); (2) reduced insulin secretion [insulinogenic index at 30 min p = 0.02, disposition index (D I) p = 0.03] in control participants during an OGTT; (3) reduced second-phase insulin secretion at 30 min (p = 0.04) and 60 min (p = 0.02) during an IVGTT; and (4) reduced D I over time in the Botnia prospective cohort (p = 0.05).
Conclusions/interpretation
We conclude that genetic variation in the CACNA1E gene contributes to an increased risk of the development of type 2 diabetes by reducing insulin secretion.
Similar content being viewed by others
Introduction
Type 2 diabetes is a common multifactorial disorder, characterised by chronic hyperglycaemia resulting from incapacity of beta cells to compensate for the degree of insulin resistance [1]. Thus, genes regulating insulin secretion are plausible candidate genes for type 2 diabetes. To date, genetic variants in TCF7L2, KCNJ11, TCF14 (also known as HNF-4α), TCF1 and the newly discovered genes HHEX, CDKAL1, CDKN1A/B and IGF2BP2 have been shown to influence insulin secretion and risk of type 2 diabetes [2–11].
Glucose-stimulated insulin secretion is biphasic. Impaired, or even completely absent first-phase insulin secretion is an early feature of type 2 diabetes, whereas second-phase insulin secretion deteriorates with progression of the disease. Data from rodent models suggest that first-phase insulin secretion results from exocytosis of insulin granules already present at the plasma membrane, whereas second-phase secretion involves recruitment of insulin granules from the beta cell interior [12, 13]. Triggering of biphasic insulin secretion requires generation of beta cell electrical activity, which results from a functional interplay between the beta cell ATP-sensitive K+ channel (which, in part, is encoded by the KCNJ11 gene) and voltage-dependent Ca2+ channels (VDCC) [14]. Pancreatic beta cells express several types of VDCCs in humans and rodents, including the L-type CaV1.2 and R-type CaV2.3 [15, 16]. Studies in rodent models have demonstrated that these channels play distinct roles for the characteristic biphasic insulin release pattern and while CaV1.2 evokes the first phase [17], the CaV2.3 is considered responsible for sustained second-phase insulin release [18]. Mice lacking the CaV2.3 channel suffer from fasting hyperglycaemia and reduced glucose clearance [19, 20].
The human CaV2.3 pore-forming subunit is encoded by CACNA1E and maps to chromosome 1q25-q31, which is telomeric to a highly replicated region for linkage to type 2 diabetes in humans [21–25]. Taken together these reports suggest that the CaV2.3 channel could play an important role in regulation of insulin secretion, particularly second-phase insulin secretion, thus CACNA1E is a good candidate gene for type 2 diabetes.
To test the hypothesis that variation in CACNA1E increases the risk of type 2 diabetes, we investigated whether single nucleotide polymorphisms (SNPs) in this gene: (1) were associated with type 2 diabetes in 3,592 type 2 diabetes cases and 4,455 healthy control participants; (2) had an effect on insulin secretion in 935 individuals during an IVGTT; (3) predicted future type 2 diabetes; or (4) changed glucose and insulin concentrations over time in 2,293 individuals from Botnia who were followed for 6 years.
Methods
Patients, case–control studies
Botnia
A case–control sample consisting of 752 persons with type 2 diabetes and 715 healthy control participants from the Botnia study was used to screen for several SNPs in CACNA1E [26, 27] (Table 1). Type 2 diabetes was diagnosed according to WHO criteria [1], with C-peptide concentrations ≥0.3 nmol/l, no GAD antibodies and age at onset >35 but <75 years of age. The control persons had fasting plasma glucose below 6.0 mmol/l and no first or second-degree family history of type 2 diabetes.
Malmo
The case–control sample used for replication included 2,830 Scandinavian patients with type 2 diabetes (from a local Diabetes Registry in Malmo [28]) and 3,740 unrelated ethnically matched healthy control persons (from the Malmo Diet and Cancer Study [29]; Table 1). Type 2 diabetes was diagnosed according to WHO criteria [1] with C-peptide concentrations ≥0.3 nmol/l, no GAD antibodies and age at onset >35 but <75 years of age. The control participants had fasting blood glucose levels below 5.5 mmol/l, HbA1c <6.0% and no known first-degree relative with diabetes. Participants with type 1 diabetes or genetically verified MODY were excluded from both studies.
Patients, prospective study
The Botnia study was initiated in 1990 and is a family-based study seeking to identify genes that increase susceptibility to type 2 diabetes [26]. Non-diabetic participants, who were either family members of type 2 diabetes patients or controls or spouses without family history of type 2 diabetes and aged between 18 and 70 years, were invited to prospective visits every 2 to 3 years. Of them, 2,293 non-diabetic individuals (1,051 men/1,242 women) underwent repeated OGTTs every 2–3 years during a median period of 6 years (Table 1) [26]. Of them, 132 developed type 2 diabetes. All subjects gave their informed consent to the study, which was approved by local ethics committees.
Measurements including assessment of first- and second-phase insulin secretion
The participants’ weight, height, waist and hip circumference, fat-free mass and blood pressure were measured as previously reported [26]. In the Botnia study, blood samples were drawn at −10, 0, 30, 60 and 120 min of the OGTT for measurement of plasma glucose (mmol/l) and serum insulin concentrations (pmol/l). Insulin resistance was estimated according to the homeostasis model assessment (HOMA) index [30]. Beta cell function was estimated as insulinogenic index (I/G 30; i.e. insulin 30 min–fasting insulin/glucose 30 min; pmol/mmol) and disposition index (D I; i.e. [I/G 30]/HOMA), which is a measure of beta cell function corrected for insulin resistance [31]. HOMA-β was calculated as previously described [32].
From the Botnia study, 935 individuals [26] underwent an IVGTT with measurements of glucose (mmol/l) and insulin (pmol/l) concentrations at −10, 0, 2, 4, 6, 8, 10, 20, 30, 40, 50, 60 min of the IVGTT. Of these, 463 had normal glucose tolerance, 272 had impaired fasting glucose or impaired glucose tolerance and 136 had type 2 diabetes; 599 (63%) of the 935 undergoing IVGTT also participated in the prospective study. First-phase insulin secretion was assessed from 0–10 min and a crude assessment of second-phase insulin secretion (SPIS) between 10 and 60 min was done (Table 1). Since SPIS was estimated during declining glucose concentrations in the above 935 individuals, we also assessed SPIS in 46 of the 935 individuals by means of hyperglycaemic clamp with measurements of glucose (mmol/l) and insulin (pmol/l) at −10, 0, 2, 4, 6, 8, 10, 15, 30, 45, 60, 75, 90, 105, 120, 135 and 150 min.
Genotyping
SNPs in a region 30 kb upstream and 20 kb downstream of CACNA1E were chosen from the International Hapmap Project web site (http://www.hapmap.org, last accessed in September 2007). Haplotype tagging SNPs (htSNPs) were selected from the Hapmap data (Hapmap public release no. 16c.1, phase I) using the program TAGGER [33]. TAGGER was used with a 5% minor allele frequency cutoff, aggressive tagging (two and three marker haplotypes), a 200 kb tagging distance and r 2 = 1.0. Sixty htSNPs, three non-synonymous and four synonymous SNPs were genotyped in the case–control sample from Botnia by primer extension of multiplex products with detection by matrix-assisted laser desorption/ionisation time-of-flight (MALDI-TOF) mass spectrometry using the Homogeneous MassExtend (hME) protocol on a Sequenom platform (Sequenom, San Diego, CA, USA).
Five SNPs that were nominally associated with type 2 diabetes were followed up in the replication sample (n = 6,570) by primer extension of multiplex products with detection by MALDI-TOF mass spectrometry using the iPLEX protocol on a Sequenom platform (Sequenom). Native DNA was used for all genotyping, except for the controls in the replication sample, where whole genome amplified DNA was used (DECODE, Reykjavik, Iceland).
The five SNPs were also genotyped in the Botnia prospective cohort (n = 2,293) as well as in participants who underwent IVGTT and hyperglycaemic clamp (n = 935); genotyping was done using the allelic discrimination technique (Applied Biosystems, Foster City, CA, USA) on an Applied Biosystems 7900HT instrument according to the manufacturers’ protocol.
In the Botnia case–control sample, 67 (of 140) SNPs were chosen from the International Hapmap project (phase I 16c.1) and genotyped (Fig. 1). Of them, four markers were monomorphic, one failed Hardy–Weinberg equilibrium test (p < 0.01) and one had success rate <85%; nine assays failed genotyping and were removed from further analysis. In total, 52 SNPs were used for subsequent analysis in the Botnia case–controls. Five of them were followed up in the Malmo case–controls. Genotype frequencies in the two case–control samples were in concordance with genotype frequencies observed in CEPH (Utah residents with ancestry from northern and western Europe) individuals from the International HapMap consortium and in Hardy–Weinberg equilibrium [Electronic supplementary material (ESM) Table 1]. A high degree of concordance with <0.6% genotypes differing between native and whole genome amplified DNA was observed when testing 7,480 samples for SNPs at different chromosomes.
Linkage disequilibrium (LD) across the CACNA1E gene in the Botnia case–control sample. SNPs rs679931, rs17443123, rs4652675, rs199925 and rs473200 were chosen for genotyping in the larger replication case–control material. (This figure was prepared using LocusView, unpublished software by T. Petryshen, A. Kirby, M. Ainscow, Copyright© 2003, 2004—The Broad Institute. Available from http://www.broad.mit.edu/mpg/locusview/, last accessed in September 2007)
Statistical analysis
Power was calculated using the Genetic Power Calculator (available from http://pngu.mgh.harvard.edu/~purcell/gpc/, last accessed in September 2007) [34], where type 2 diabetes prevalence was assumed to be 6% under a dominant model with a relative risk of 1.3.
With a minor allele frequency of 5%, a type 2 diabetes prevalence of 6% and a relative risk of 1.3 at α = 0.05, we had 38% power using a dominant model in the Botnia case–control sample and a corresponding 93% in the replication case–control sample. Only results that could be replicated in the Malmo case–control sample were considered significant.
Allelic association (χ 2 test, 1 df) and linkage disequilibrium between SNPs was calculated using Haploview 3.32 [35] and D′ values were calculated with 95% CIs [36]. Multivariate logistic regression was applied to examine differences in genotype distribution for the nominally associated SNPs. Analyses were adjusted for age at onset (cases) and age at visit (controls), BMI and sex. Combined odds ratio (OR) and p values were calculated by pooling the two case–control samples. Meta-analysis was performed using a Mantel–Haenszel test.
Baseline levels and rate changes of phenotype residuals between the different genotype carriers in the Botnia study were calculated using multiple and linear regression analyses adjusted for age, BMI, sex and family history of type 2 diabetes. Standard errors were adjusted for repeated measurements from the same individual by the generalised estimating equation (GEE) method [37, 38]. The GEE model was necessary because the repeated phenotype measurements were obtained at different time points in different participants. Phenotypic data from each visit except the visit of diagnosis for the converters and all except the last visit for the non-converters were used in the analysis. This was necessary to avoid the confounding effect of overt hyperglycaemia on beta cell function, as we did not have measurements immediately before onset of diabetes.
The Cox proportional hazard model was used to estimate relative genotype and phenotype effect on the risk of developing type 2 diabetes. In conventional survival analyses all individuals would enter the study at the same time point and time in the study would be shown on the x-axis. This could confound the estimation of survival in young individuals. To circumvent this problem, we adopted an alternative approach, where the starting point of analysis equals the age of the youngest individual at entry to study and the x-axis shows the age of participants. Data were left truncated, as only disease-free individuals were included. Volunteers were followed to the date of last recording (31 December 2003). Survival analyses were stratified for sex and adjusted for family history of diabetes and BMI [39]. β-Coefficients were estimated from all observations for calculation of the variance of β, and each pedigree was treated as an independent entity (y = α + βx). Individuals with missing data for any of the covariates were excluded from the analyses.
Normally distributed continuous variables are presented as mean ± SD, whereas non-normally distributed data were logarithmically transformed before analysis and presented as median with interquartile range. A two-tailed p value of <0.05 was considered statistically significant and nominal p values for regression analysis, GEE and Cox proportional hazards model are presented. No adjustments for multiple testing were performed. Only genetic association (p < 0.05 two-tailed) that could be replicated in the follow up case–control sample was considered statistically significant. All statistical analyses were performed using STATA (StataCorp LP, College Station, TX, USA) and/or Number Cruncher Statistical Systems, version 2000 (Kaysville, UT, USA).
Results
Polymorphisms in CACNA1E are associated with type 2 diabetes
Ten of the 52 SNPs had a nominal p value of <0.05 for type 2 diabetes in the Botnia case–control sample, with the major allele for rs199925 (T allele) showing the strongest association, p allelic = 0.007. Also, the haplotype block containing rs199925 was nominally associated with type 2 diabetes (p = 0.008). However, none of the SNPs or the haplotype blocks were associated with type 2 diabetes after 10,000 permutations (Table 2; for data for all 52 SNPs, see ESM Table 1).
We screened for and fitted the best genetic model to each of the ten SNPs that showed nominal allelic association (1 df) with type 2 diabetes among the Botnia case–controls. Five of these SNPs also showed a trend to or were nominally associated with type 2 diabetes on the genotype level, with results for carriers as follows: rs679931 TT genotype OR 1.4, 95% CI 1.0–2.0, p = 0.06; rs17443123 AA genotype OR 1.4, 95% CI 1.0–1.8, p = 0.03; rs4652675 AA/AG genotypes OR 1.7, 95% CI 1.1–2.5, p = 0.009; rs199925 TT/TG genotypes OR 2.3, 95% CI 1.1–4.8, p = 0.03; and rs473200 CT/TT genotypes OR 1.3, 95% CI1.0–1.7, p = 0.02. These five nominally significant SNPs (rs679931, rs17443123, rs4652675, rs199925 and rs473200) were chosen for further genotyping in a second case–control sample consisting of 2,830 cases and 3,740 control participants from Malmo, Sweden.
Replication in the case–control sample from southern Sweden
Since replication was attempted in a new case–control sample from southern Sweden (Malmo), we genotyped three SNPs in known type 2 diabetes susceptibility genes, i.e. rs5219 (E23K) in KCNJ11, rs1801282 (P12A) in PPARG, and rs7903146 in TCF7L2. All three SNPs were associated with type 2 diabetes (OR 1.2, p = 0.0007; OR 1.1, p = 0.04; OR 1.4, p = 2.5e−17, respectively). Later, the same material was used to replicate findings from a whole genome association study carried out by the Diabetes Genetics Initiative (DGI) [9].
Individuals with the rs679931 TT genotype were at increased risk of type 2 diabetes (OR 1.2, 95% CI 1.0–1.5, p = 0.01 one-tailed vs AA/AT genotype carriers). Individuals with the rs199925 GG genotype had an OR of 1.5 (95% CI 1.1–2.1, p = 0.01 one-tailed) vs TT/TG genotype carriers. The other three SNPs were not significantly associated with type 2 diabetes in the replication case–control sample (rs17443123 OR 1.0, 95% CI 0.9–1.1, p = 0.8; rs4652675 OR 1.0, 95% CI 0.8–1.2, p = 0.8; rs473200 OR 0.8, 95% CI 0.6–1.2, p = 0.3).
The combined OR, adjusted for age, BMI and sex, for the rs679931 TT genotype in the two case–control studies was 1.3 (95% CI 1.1–1.5, p = 0.004 two-tailed) and for the rs199925 GG genotype was 0.9 (95% CI 0.2–1.2, p = 0.3).
Effects of common variants in CACNA1E on measures of insulin secretion
Control individuals from the Botnia case–control sample with type 2 diabetes associated with the rs679931 TT genotype had significantly lower I/G 30 (27.1 ± 4.0 vs 36.3 ± 1.4 pmol/mmol, p = 0.02) and D I (27.0 ± 8.1 vs 43.4 ± 2.8, p = 0.03) than individuals with the AA/AT genotypes. Individuals with the rs199925 GG genotype had significantly lower 30 min insulin (162.1 ± 72.0 vs 327.0 ± 12.0 pmol/l, p = 0.01), I/G 30 (17.2 ± 8.5 vs 36.0 ± 1.4 pmol/mmol), p = 0.01) and HOMA-β (51.4 ± 18.1 vs 81.2 ± 3.1, p = 0.03) than individuals with the T allele. These two markers were not in linkage disequilibrium (D′ = 0.02, r 2 = 0.0). No glucose or insulin secretion differences were observed among the control persons for the other three tested polymorphisms.
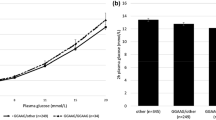
The five SNPs were also tested for their effects on changes in insulin secretion over time in 2,293 individuals from the Botnia prospective study (Table 1). Individuals with the rs679931 TT genotype had decreased D I (p = 0.05) over time compared with individuals with the AA/AT genotypes. Additionally these SNPs were genotyped in individuals with an IVGTT; non-type 2 diabetes carriers of the rs679931 TT genotype had significantly reduced SPIS as judged from lower insulin concentrations at 30 min (100.1 ± 8.8 vs 122.0 ± 3.3 pmol/l, p = 0.04) and 60 min (79.4 ± 8.9 vs 103.2 ± 3.4 pmol/l, p = 0.02; Fig. 2).
During the IVGTT, individuals with the rs679931 TT genotype (white circles) had significantly reduced insulin secretion at 30 min (p = 0.04) and 60 min (p = 0.02) as compared with individuals with the AA/AT genotypes (black circles). The strongest reduction in insulin secretion was observed for TT genotype carriers during second-phase insulin secretion (30–60 min). This reduction would be compatible with an effect on second-phase insulin secretion. *p < 0.05
In the Botnia prospective cohort, 132 of the 2,293 individuals converted to type 2 diabetes during the median follow-up of 6 years [39]. None of the five SNPs conferred an increased risk of future type 2 diabetes, with hazard ratios (HR) as follows in this population: rs679931 HR 1.4, 95% CI 0.8–2.3, p = 0.2; rs17443123 HR 1.3, 95% CI 0.8–2.0, p = 0.3; rs4652675 HR 1.8, 95% CI 0.7–3.2, p = 0.3; rs199925 HR 1.6, 95% CI 0.5–5.7, p = 0.5; and rs473200 HR 1.0, 95% CI 0.7–1.1, p = 0.9. However, given the low number of converters, the power to detect future risk of type 2 diabetes was limited.
During the course of this study several whole genome-wide scans for type 2 diabetes have been published [7–11]. Thus, we looked closer at the CACNA1E locus in the publicly available DGI and the Wellcome Trust Case Control Consortium (WTCCC) genome-wide scans (ESM Table 2) [8, 9]. There was no difference in allele frequency between cases and controls for the SNP rs679931 in these two scans (p allelic = 0.3 and 0.4, respectively). However, a meta-analysis combining data from the present study with data from the DGI and WTCCC genome-wide scans showed an association between the rs679931 TT genotype and type 2 diabetes (OR 1.2, 95% CI 1.1–1.3, p = 0.002).
Discussion
Glucose-stimulated insulin secretion is dependent upon the interaction between KATP channels, voltage-gated Ca2+ channels and pumps, as well as glucose metabolism in the beta cell. The E23K polymorphisms in the KCNJ11 gene, which encodes the Kir6.2 subunit of the KATP channel, has recently been associated with type 2 diabetes [2], suggesting that genes encoding other ion channels are possible candidate genes for type 2 diabetes. Based on this knowledge and given that CACNA1E resides in a highly replicated region for type 2 diabetes (chromosome 1q25-q31) and that rodent models lacking the CaV2.3 channel display reduced second-phase insulin release and impaired glucose homeostasis, we investigated SNPs in the CACNA1E gene for association with type 2 diabetes. We found that rs673391 (TT genotype) located in the CACNA1E gene was associated with (1) type 2 diabetes in two independent case–control samples (p = 0.06, p = 0.01 one-tailed, p = 0.004 in the combined sample); (2) reduced insulin secretion in response to oral (I/G 30 p = 0.02, D I p = 0.03) as well as to intravenous (p = 0.02) glucose, particularly the second phase; and (3) a decline in insulin secretion over a 6-year period (D I p = 0.05).
To evaluate common variation in CACNA1E and risk of type 2 diabetes and defects in insulin secretion we used: (1) a two-phase case–control strategy; (2) a prospective cohort; and (3) individuals who had undergone an IVGTT. A total of 52 SNPs were successfully genotyped and screened for association with type 2 diabetes in patients and healthy control participants from the Botnia region, Finland. Five of these SNPs were found to be associated with type 2 diabetes on the genotype level and were further analysed in a second sample of patients and healthy control persons from Malmo, Sweden. Due to the limited power in the screening material (Botnia), and to reduce the risk of false positive findings, we only considered results that were also significantly associated with type 2 diabetes in the larger case–control sample from Malmo. Only the rs679931 TT genotype met these requirements. Further analyses of these five SNPs showed that the rs679931 TT genotype was associated with impaired insulin secretion during OGTT and IVGTT. Interestingly, insulin secretion was significantly reduced among non-type 2 diabetes individuals during the late phase (30–60 min) of the IVGTT, which is comparable with the reduced second-phase glucose-stimulated insulin secretion observed in INS-1 cells and in CaV2.3(−/−) mice [18, 20, 40]. Also, the TT genotype carriers showed a decline in insulin secretion adjusted for insulin resistance (D I) over a 6-year period. Reduced beta cell function is known to be a strong risk factor for future type 2 diabetes [41], and we investigated the related risk for future type 2 diabetes in the Botnia prospective cohort. Although the reduced insulin secretion was not translated into a significantly increased risk of future type 2 diabetes, there was a trend towards an increased risk (HR = 1.4, p = 0.1 one-tailed) for individuals with the rs673391 TT genotype. However, with few converters (132 of 2,293 converted to type 2 diabetes during the 6-year follow-up) the power was limited and thus it would be difficult to detect association between a genetic variant with a modest effect size on a polygenic disease like type 2 diabetes. The limited size of the prospective study also increases the risk of detecting false positive associations, but since the observed trend for the rs679931 is concordant with the findings from our case–control samples, we believe this observation is the result of a small amount of material that lacks power, rather than of a false positive observation.
Since we introduced a new large case–control sample from southern Sweden, we genotyped three known type 2 diabetes associated SNPs, i.e. rs5219 (E23K) in the KCNJ11, the rs1801282 (P12A) in the PPARG and the rs7903146 in the TCF7L2 genes for validation. All three genes showed association with type 2 diabetes with the most striking for TCF7L2 (p = 2.5e−17). As with the hereto identified type 2 diabetes variants (TCF7L2 excluded) the strength of the association between rs679931 and type 2 diabetes was modest.
Here, we suggest that variants in CACNA1E act to impair insulin secretion via reduced expression or function of the CaV2.3 protein in the beta cells. However, since we had no measurements of beta cell mass, one could speculate that increased expression or activity of CaV2.3 could lead to an increased influx of Ca2+ in the beta cells, potentially increasing cell death. This would also result in reduced insulin secretion over time, as observed in the Botnia prospective cohort (D I p = 0.05), as well as in an increased risk of developing type 2 diabetes.
Although we observed association with type 2 diabetes between several of the tested SNPs in CACNA1E, a potential weakness of our study is the low power finding association in the Botnia case–control sample for SNPs with a relatively low frequency (5%). However, power to detect association increases from 38% to 56% at a minor allele frequency of as little as 10%. Even though we replicated the results for rs679931 TT genotype in a large case–control sample with >93% power and showed decreased insulin secretion for individuals with the rs679931 TT genotype, we cannot exclude the possibility of false-positive study-wide results in our study, given the number of tests performed at α = 0.05. Encouragingly, in a meta-analysis combining our screening and replication samples with data from the DGI and WTCCC genome-wide scans [8, 9], the rs679931 TT genotype was associated with type 2 diabetes, p = 0.002, suggesting that variation in the CACNA1E gene could indeed increase risk of type 2 diabetes. Future work investigating the association between variation in CACNA1E and both type 2 diabetes and insulin secretion defects is warranted in large and ethnically distinct study groups.
In conclusion, genetic variants in the CACNA1E gene (CaV2.3) are associated with type 2 diabetes and reduced insulin secretion, particularly second-phase insulin secretion.
Abbreviations
- DGI:
-
Diabetes Genetics Initiative
- D I :
-
disposition index (insulin)
- GEE:
-
generalised estimating equation
- HOMA:
-
homeostasis model assessment
- HR:
-
hazard ratio
- HtSNP:
-
haplotype tagging SNP
- I/G 30 :
-
insulinogenic index
- MALDI-TOF:
-
matrix-assisted laser desorption/ionisation time-of-flight
- OR:
-
odds ratio
- SNP:
-
single nucleotide polymorphism
- SPIS:
-
second-phase insulin secretion
- VDCC:
-
voltage-dependent Ca2+ channel
- WTCCC:
-
Wellcome Trust Case Control Consortium
References
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553
Gloyn AL, Weedon MN, Owen KR et al (2003) Large-scale association studies of variants in genes encoding the pancreatic beta-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) confirm that the KCNJ11 E23K variant is associated with type 2 diabetes. Diabetes 52:568–572
Grant SFA, Thorleifsson G, Reynisdottir I et al (2006) Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat Genet 38:320–323
Holmkvist J, Cervin C, Lyssenko V et al (2006) Common variants in HNF-1a and risk of type 2 diabetes. Diabetologia 49:2882–2891
Silander K, Mohlke KL, Scott LJ et al (2004) Genetic variation near the hepatocyte nuclear factor-4{alpha} gene predicts susceptibility to type 2 diabetes. Diabetes 53:1141–1149
Love-Gregory LD, Wasson J, Ma J et al (2004) A common polymorphism in the upstream promoter region of the hepatocyte nuclear factor-4{alpha} gene on chromosome 20q is associated with type 2 diabetes and appears to contribute to the evidence for linkage in an Ashkenazi Jewish population. Diabetes 53:1134–1140
Sladek R, Rocheleau G, Rung J et al (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Zeggini E, Weedon MN, Lindgren CM et al (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science (New York, NY) 316:1336–1341
Diabetes Genetics Initiative of Broad Institute of Harvard and MIT LU, and Novartis Institutes of BioMedical Research, Saxena R, Voight BF et al (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Steinthorsdottir V, Thorleifsson G, Reynisdottir I et al (2007) A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet 39:770–775
Scott LJ, Mohlke KL, Bonnycastle LL et al (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345
Ohara-Imaizumi M, Nakamichi Y, Nishiwaki C, Nagamatsu S (2002) Transduction of MIN6 beta cells with TAT-syntaxin SNARE motif inhibits insulin exocytosis in biphasic insulin release in a distinct mechanism analyzed by evanescent wave microscopy. J Biol Chem 277:50805–50811
Ivarsson R, Jing X, Waselle L, Regazzi R, Renstrom E (2005) Myosin 5a controls insulin granule recruitment during late-phase secretion. Traffic 6:1027–1035
Kanno T, Rorsman P, Gopel SO (2002) Glucose-dependent regulation of rhythmic action potential firing in pancreatic beta-cells by KATP-channel modulation. J Physiol 545:501–507
Schneider T, Wei X, Olcese R et al (1994) Molecular analysis and functional expression of the human type E neuronal Ca2+ channel alpha 1 subunit. Recept Channels 2:255–270
Shao-Nian Y, Per-Olof B (2006) The role of voltage-gated calcium channels in pancreatic beta-cell physiology and pathophysiology. Endocr Rev 27:621–676
Schulla V, Renstrom E, Feil R et al (2003) Impaired insulin secretion and glucose tolerance in beta cell-selective Ca(v)1.2 Ca2+ channel null mice. EMBO J 22:3844–3854
Jing X, Li D-Q, Olofsson CS et al (2005) CaV2.3 calcium channels control second-phase insulin release. J Clin Invest 115:146–154
Matsuda Y, Saegusa H, Zong S, Noda T, Tanabe T (2001) Mice lacking Cav2.3 ([alpha]1E) calcium channel exhibit hyperglycemia. Biochem Biophys Res Commun 289:791–795
Pereverzev A, Mikhna M, Vajna R et al (2002) Disturbances in glucose-tolerance, insulin-release, and stress-induced hyperglycemia upon disruption of the Cav2.3 ({alpha}1E) subunit of voltage-gated Ca2+ channels. Mol Endocrinol 16:884–895
Xiang K, Wang Y, Zheng T et al (2004) Genome-wide search for type 2 diabetes/impaired glucose homeostasis susceptibility genes in the Chinese: significant linkage to chromosome 6q21-q23 and chromosome 1q21-q24. Diabetes 53:228–234
Wiltshire S, Hattersley AT, Hitman GA et al (2001) A genomewide scan for loci predisposing to type 2 diabetes in a U.K. population (the Diabetes UK Warren 2 Repository): analysis of 573 pedigrees provides independent replication of a susceptibility locus on chromosome 1q. Am J Hum Genet 69:553–569
Vionnet N, Hani E-H, Dupont S et al (2000) Genomewide search for type 2 diabetes-susceptibility genes in French whites: evidence for a novel susceptibility locus for early-onset diabetes on chromosome 3q27-qter and independent replication of a type 2-diabetes locus on chromosome 1q21-q24. Am J Hum Genet 1470–1480
Ng MCY, So W-Y, Cox NJ et al (2004) Genome-wide scan for type 2 diabetes loci in Hong Kong Chinese and confirmation of a susceptibility locus on chromosome 1q21-q25. Diabetes 53:1609–1613
Hsueh W-C, St. Jean PL, Mitchell BD et al (2003) Genome-wide and fine-mapping linkage studies of type 2 diabetes and glucose traits in the old order Amish: Evidence for a new diabetes locus on chromosome 14q11 and confirmation of a locus on chromosome 1q21-q24. Diabetes 52:550–557
Groop L, Forsblom C, Lehtovirta M et al (1996) Metabolic consequences of a family history of NIDDM (the Botnia Study): evidence for sex-specific parental effects. Diabetes 45:1585–1593
Tripathy D, Carlsson M, Almgren P et al (2000) Insulin secretion and insulin sensitivity in relation to glucose tolerance: lessons from the Botnia Study. Diabetes 49:975–980
Lindholm E, Agardh E, Tuomi T, Groop L, Agardh CD (2001) Classifying diabetes according to the new WHO clinical stages. Eur J Epidemiol 17:983–989
Berglund G, Elmstahl S, Janzon L, Larsson S (1993) The Malmo diet and cancer study. Design and feasibility. J Intern Med 233(1):45–51
Levy J, Matthews D, Hermans M (1998) Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care 21:2191–2192
Bergman R, Phillips LS, Cobelli C (1981) Physiologic evaluation of factors controlling glucose tolerance in man: measurement of insulin sensitivity and beta-cell glucose sensitivity from the response to intravenous glucose. J Clin Invest 68:1456–1467
Gonzalez-Ortiz M, Martinez-Abundis E (2003) Comparison of several formulas to assess insulin action in the fasting state with the hyperglycemic–hyperinsulinemic clamp technique in healthy individuals. Rev Invest Clin 55:419–422
de Bakker PIW, Yelensky R, Pe’er I, Gabriel SB, Daly MJ, Altshuler D (2005) Efficiency and power in genetic association studies. Nat Genet 37:1217–1223
Purcell S, Cherny SS, Sham PC (2003) Genetic power calculator: design of linkage and association genetic mapping studies of complex traits. Bioinformatics 19:149–150
Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263–265
Gabriel SB, Schaffner SF, Nguyen H et al (2002) The structure of haplotype blocks in the human genome. Science 296:2225–2229
Liang K, Zeger S (1986) Longitudinal data analysis using generalized linear models. Biometrica 73:13–22
Liang K, Zeger S (1993) Regression analysis for correlated data. Annu Rev Public Health 14:43–68
Lyssenko V, Almgren P, Anevski D et al (2005) Genetic prediction of future type 2 diabetes. PLoS Med 2:e345
Vajna R, Klockner U, Pereverzev A et al (2001) Functional coupling between ‘R-type’ Ca2+ channels and insulin secretion in the insulinoma cell line INS-1. Eur J Biochem 268:1066–1075
Pimenta W, Korytkowski M, Mitrakou A et al (1995) Pancreatic beta-cell dysfunction as the primary genetic lesion in NIDDM. Evidence from studies in normal glucose-tolerant individuals with a first-degree NIDDM relative. JAMA 273:1855–1861
Acknowledgements
This work was supported by grants from: the Swedish Research Council, including a Linneaus grant; the Novo Nordisk Foundation; The Sigrid Juselius Foundation; The Folkhälsan Research Foundation; and the Lundberg Foundation. Genotyping using Sequenom was performed at Swegene PPD (http://www.swegene.org). We thank all participants for making this project possible.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Holmkvist, J., Tojjar, D., Almgren, P. et al. Polymorphisms in the gene encoding the voltage-dependent Ca2+ channel CaV2.3 (CACNA1E) are associated with type 2 diabetes and impaired insulin secretion. Diabetologia 50, 2467–2475 (2007). https://doi.org/10.1007/s00125-007-0846-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0846-2