Abstract
Aims/hypothesis
In case-control studies, polymorphisms at the atrial natriuretic peptide gene (ANP) locus have been associated with presence of albuminuria in Type 1 and Type 2 diabetes. We evaluated the relationship between the ScaI and BstxI polymorphisms and albuminuria in the general population of the Mexico City Diabetes Study.
Methods
Allele/genotype frequencies were analysed by PCR-RFLP analysis using ScaI (wild, A 2 vs mutated, A 1) and BstxI (wild, C 708 vs mutated, T 708) enzyme.
Results
Among 1288 subjects, hypertension was present in 112 subjects, Type 2 diabetes in 191 and impaired glucose tolerance in 136; microalbuminuria was present in 464 subjects, and clinical proteinuria in 199. General frequencies were 0.93 and 0.96 for the wild alleles, and 0.07 and 0.04 for the mutated alleles, respectively for ScaI and BstxI. Frequency of A 1was 0.08 in normoalbuminuric, 0.05 in microalbuminuric, and 0.05 in proteinuric patients (χ2=7.3, p=0.025). Frequency of T 708 was 0.06 in normoalbuminuric and 0.03 microalbuminuric and 0.03 in proteinuric subjects (χ 2=8.1, p=0.017). By multivariate analysis, the associations between A 1 or T 708 allele and albuminuria were independent of age, sex, BMI, diabetes, and hypertension, (odds ratio (OR) 0.60, p=0.01, (OR) 0.51, p=0.004, respectively).
Conclusion/interpretation
In the general population of Mexico City, both polymorphisms of ANP are associated with albuminuria independently of hypertension, and could play a role in protecting subjects against development of albuminuria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Diabetic nephropathy occurs in familial clusters, indicating that genetic factors are involved [1]. The genes responsible for the predisposition to kidney disease in diabetic subjects are still unknown, but the atrial natriuretic peptide gene (ANP) is regarded as a plausible candidate.
ANP is an endogenous vasoactive peptide, produced mainly in cardiac atria, which plays an important role in blood pressure regulation by modulating sodium homeostasis and the renin-angiotensin-aldosterone system [2]. ANP also affects renal haemodynamics and microvascular permeability to macromolecules [3]. In transgenic animals, disruption of ANP leads to salt-sensitive hypertension [4]; this finding, together with the availability of reported genetic markers at the ANP locus [5], has prompted intense investigation. However, the results of association studies between polymorphisms at the ANP locus and hypertension [5] or diabetic nephropathy [6] have been conflicting. In Type 1 and Type 2 diabetic patients [7] we found an independent association between ScaI and BstxI polymorphisms of ANP and the presence of albuminuria, and we suggested that ANP variants could exert a protective effect against the development and/or progression of kidney damage in diabetes. Similar evidence from population-based observations is, however, lacking. The aim of our study therefore was to evaluate the role of ANP in the development of albuminuria in the Mexico City Diabetes Study [8], a population with a high prevalence of Type 2 diabetes.
Materials and Methods
Subjects
Data were collected as part of the Mexico City Diabetes Study [8], a population-based survey of diabetes and cardiovascular disease. Among 2272 subjects completing medical examination at the clinic, DNA was available from 1288 subjects.
The protocol was approved by the Institutional Review Board of the University of Texas Health Science Center at San Antonio and the American British Cowdray Hospital in Mexico City, and all subjects gave informed consent.
Physical measurements
Height, weight, waist and hip circumferences, BMI and systolic and diastolic blood pressures were measured as described elsewhere [8].
Blood specimens
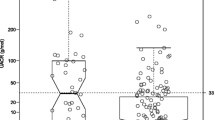
All participants were asked to fast for at least 12 h before the examination. Blood was obtained in the fasting state and 2 h after a standardised 75-g oral glucose load. Fasting concentrations of serum insulin (FPI), plasma glucose (FPG), total cholesterol (TC), LDL, HDL, and triglyceride (TG), and glucose (2-h PG) and insulin (2-h PI) concentrations 2 h post-glucose were measured as described elsewhere [8]. At baseline, subjects were classified as having IGT or Type 2 diabetes according to the American Diabetes Association criteria [9]. Both clinical proteinuria and microalbuminuria were assessed in early-morning urine samples from 994 subjects [8] at the time of their clinical examination. Subjects were considered to be positive for clinical proteinuria (CP) if they had a 1+ or greater reaction to Albustix (Ames, Elkhart, Ind., USA). Microalbuminuria (mA) was measured by a semiquantitative technique (Microalbumin test tablet, Ames). Subjects were considered to be positive if they had a 1+ or greater reaction (approximately equal to 40 mg/l or above).
Screening for polymorphisms by PCR fragment length polymorphism (RFLP)
ANP, located on the short arm of chromosome 1, contains three exons separated by two introns. A fragment of 640 base-pairs (bp), (exon I-II), was analysed by PCR-RFLP, as described elsewhere [10]. In wild-type samples (C 708), BstxI identified a single restriction site inside the PCR products, and gave origin to fragments of 442 and 198 bp. If the point mutation was present, BstxI yielded fragments of 262, 198, 180 bp in homozygotes (T 708).
A fragment of 133 bp (intron 2 and the 3' flanking region) of ANP was analysed by PCR-RFLP, as described elsewhere [10]. In wildtype subjects, after digestion with ScaI restriction enzyme, two fragments of 77 and 56 bp were generated (A 2 allele) and in the absence of the site a fragment of 133 bp was observed (A 1 allele).
Statistical analysis
Data are presented as means ± SE. Categorical variables were compared by a chi-square test. Multiple associations were tested by using general linear models including both continuous and categorical variables; results are expressed as the odds ratio (OR) with 95% confidence intervals (CI). Genetic data are presented according to both genotype and allele frequency. The chi-square analysis was used to test for Hardy-Weinberg equilibrium within the three main groups of study subjects (Type 2 diabetes, IGT, non-diabetic controls). Differences in genotype and allele frequency across study groups were tested by Fisher's exact test and chi-square analysis, respectively.
Results
The prevalence of hypertension was higher in subjects with clinical proteinuria as compared to normoalbuminuric or microalbuminuric subjects (p<0.003) (Table 1). Similarly, stroke was more frequent in subjects with clinical proteinuria (p<0.03), whereas no association was observed between prevalence of myocardial infarction and albuminuria (p=NS).
The distributions of the ScaI and BstxI genotypes were in Hardy-Weinberg equilibrium when examined in NGT, IGT, or Type 2 diabetic groups. ScaI and BstxI genotypes were in linkage disequilibrium (p<0.0001). The genotype distributions and allele frequencies of both polymorphisms were similar in subjects stratified by glucose tolerance status. When allele frequencies were analysed according to the presence of hypertension, the frequency of the A 1 allele was higher in hypertensive as compared to normotensive subjects, whereas no such difference was found in the distribution of the T 708 allele (Table 2). Frequencies of both the A 1 allele and the T 708 allele were lower in subjects with either microalbuminuria or clinical proteinuria compared to normoalbuminuric subjects. No association was found for either polymorphism with stroke or myocardial infarction. When testing the association between ScaI polymorphism and albuminuria in diabetic and non-diabetic subjects separately, an association was found in diabetic patients, while the association was weaker among non-diabetic subjects (χ2=3.1, p=0.07).
To test for independent associations between hypertension or albuminuria and genotypes, we did a multiple logistic regression analysis in which the presence of albuminuria (microalbuminuria and clinical proteinuria) was the dependent variable, and age, sex BMI, diabetes, hypertension, and A 1 (or T 708) allele were main effects. Using this approach, the mutated ScaI genotypes emerged as an independent protective factor for micro/clinical proteinuria (OR=0.60 [0.41–0.89], p=0.01). Among the other variables, only the presence of diabetes (OR=2.02, [1.3–3.1], p=0.0046) was independently associated with albuminuria. When the mutated BstxI genotypes replaced ScaI in the same model, they also showed an independent protective effect for microalbuminuria and macroalbuminuria (OR=0.51 [0.32–0.80], p=0.004).
Discussion
We found the association between the ScaI and BstxI polymorphisms and albuminuria at the population level. ANP has long been listed as a candidate gene for familial susceptibility to hypertension and diabetic nephropathy [5, 6]. In previous studies carried out in Type 1 or Type 2 diabetic patients [7], as well as in non-diabetic patients with essential hypertension, we found that the A 1 allele was less frequent in macroalbuminuric patients as compared to normoalbuminuric subjects, while the T 708 allele was more frequent in subjects with long-term microalbuminuria. On this basis, we suggested that ANP per se could play a role in the development of albuminuria. We analysed the distribution of both ANP polymorphisms in a random sample of a whole population, in which the prevalence of microalbuminuria was apparently high also in non-diabetic subjects [8] and we confirmed the association between ANP variants and the presence of albuminuria.
The frequency of the A 1 allele varies widely depending on ethnicity [5]. However, although the frequency of the ScaI polymorphism differs in different ethnic groups, its association with albuminuria persists in both Caucasian and Mexican subjects. On the other hand, the association of ANP polymorphisms with hypertension has been inconsistent in different populations [5]. In the present study, the A 1 allele was more frequent in hypertensive than normotensive subjects, but in multivariate analysis the mutated genotypes were no longer independently associated with hypertension. On this basis, there is no clear evidence that the ANP gene is significantly involved in the pathogenesis of hypertension, whereas it seems to play a role in the development of albuminuria.
Certain lines of evidence indicate that ANP affects renal haemodynamics by raising intraglomerular pressure and modifies renal excretory function and vascular permeability in diabetes [2, 3]. With regard to the functional significance of ANP variants, in a previous study in Type 1 diabetes [10] the T 708and A 1 alleles were associated with lower circulating ANP concentrations and preserved microvascular permeability (as judged from the albumin transcapillary escape rate) independently of the presence of microalbuminuria. On these grounds, we hypothesised a protective role of the two variants in the development and/or progression of microvascular damage. The precise functional significance of BstxI and ScaI variants is currently not clear. The A 1 allele causes loss of the regular stop codon, leading to the transcription of two additional arginine residues [5]; however, the effects of these two variants on the synthesis and/or activity of the mature peptide are not known.
Overall, our findings are compatible with a role of ANP variants in protecting against the development of albuminuria also in a general population. Longitudinal studies are needed to clarify the role of this gene in the development and/or progression of albuminuria.
Abbreviations
- ANP:
-
Atrial natriuretic peptide
- NA:
-
normoalbuminuria
- mA:
-
microalbuminuria
- CP:
-
clinical proteinuria
- FPI:
-
fasting concentrations of serum insulin
- FPG:
-
fasting plasma glucose
- TC:
-
total cholesterol
- HT:
-
hypertension
- OR:
-
odds ratio
References
Quinn M, Angelico MC, Warram JH, Krolewski AS (1996) Familial factors determine the development of diabetic nephropathy in patients with IDDM. Diabetologia 39:940–945
Levin ER, Gardner DG, Samson WK (1998) Natriuretic peptides. N Engl J Med 339:321–328
Zietse R, Derkx FH, Weimar W, Schalekamp MA (1995) Effect of atrial natriuretic peptide on renal and vascular permeability in diabetes mellitus. J Am Soc Nephrol 5:2057–2066
Koh GY, Klug MG, Field LJ (1993) Atrial natriuretic factor and transgenic mice. Hypertension 22:634–639
Kato N, Sugiyama T, Morita H et al. (2000) Genetic analysis of atrial natriuretic peptide gene in essential hypertension. Clin Sci (Colch) 98:251–258
Schimdt S, Bluthner M, Giessel R et al. (1998) A polymorphism in the gene for the atrial natriuretic peptide and diabetic nephropathy. Diabetic Nephropathy Study Group. Nephrol Dial Transplant 13:1807–1810
Nannipieri M, Manganiello M, Pezzatini A, De Bellis A, Seghieri G, Ferrannini E (2001) Polymorphisms in the hANP (human atrial natriuretic peptide) gene, albuminuria, and hypertension. Hypertension 37:1416–1422
Haffner SM, Gonzales C, Valdez RA et al. (1993) Is microalbuminuria part of the prediabetic state? The Mexico City Diabetes Study. Diabetologia 36:1002–1006
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (2002) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 25: S5-S20
Nannipieri M, Penno G, Pucci L et al. (1999) Pronatriodilatin gene polymorphisms, microvascular permeability, and diabetic nephropathy in type 1 diabetes mellitus. J Am Soc Nephrol 10:1530–1541
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nannipieri, M., Posadas, R., Williams, K. et al. Association between polymorphisms of the Atrial Natriuretic Peptide gene and proteinuria: a population-based study. Diabetologia 46, 429–432 (2003). https://doi.org/10.1007/s00125-003-1047-2
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-003-1047-2