Abstract
Key message
A point mutation of RPM1 triggers persistent immune response that induces leaf premature senescence in wheat, providing novel information of immune responses and leaf senescence.
Abstract
Leaf premature senescence in wheat (Triticum aestivum L.) is one of the most common factors affecting the plant's development and yield. In this study, we identified a novel wheat mutant, yellow leaf and premature senescence (ylp), which exhibits yellow leaves and premature senescence at the heading and flowering stages. Consistent with the yellow leaves phenotype, ylp had damaged and collapsed chloroplasts. Map-based cloning revealed that the phenotype of ylp was caused by a point mutation from Arg to His at amino acid 790 in a plasma membrane–localized protein resistance to Pseudomonas syringae pv. maculicola 1 (RPM1). The point mutation triggered excessive immune responses and the upregulation of senescence- and autophagy-associated genes. This work provided the information for understanding the molecular regulatory mechanism of leaf senescence, and the results would be important to analyze which mutations of RPM1 could enable plants to obtain immune activation without negative effects on plant growth.





Similar content being viewed by others
References
Bella J, Hindle KL, McEwan PA, Lovell SC (2008) The leucine-rich repeat structure. Cell Mol Life Sci 65:2307–2333
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinski J, Ishizaki K, Leaver CJ (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42:567–585
Buchner P, Hawkesford MJ (2014) Complex phylogeny and gene expression patterns of members of the NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family (NPF) in wheat. J Exp Bot 65:5697–5710
Cao B, Ge L, Zhang M, Li F, Zhou X (2023) Geminiviral C2 proteins inhibit active autophagy to facilitate virus infection by impairing the interaction of ATG7 and ATG8. J Integr Plant Biol 65:1328–1343
Chen YM, Song WJ, Xie XM, Wang ZH, Guan PF, Peng HR, Jiao YN, Ni ZF, Sun QX, Guo WL (2020) A Collinearity-incorporating homology inference strategy for connecting emerging assemblies in the Triticeae tribe as a pilot practice in the Plant Pangenomic Era. Mol Plant 13:1694–1708
Chung EH, da Cunha L, Wu AJ, Gao Z, Cherkis K, Afzal AJ, Mackey D, Dangl JL (2011) Specific threonine phosphorylation of a host target by two unrelated type III effectors activates a host innate immune receptor in plants. Cell Host Microbe 9:125–136
Coll NS, Smidler A, Puigvert M, Popa C, Valls M, Dangl JL (2014) The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: functional linkage with autophagy. Cell Death Differ 21:1399–1408
Desmond OJ, Edgar CI, Manners JM, Maclean DJ, Schenk PM, Kazan K (2005) Methyl jasmonate induced gene expression in wheat delays symptom development by the crown rot pathogen Fusarium pseudograminearum. Physiol Mol Plant Pathol 67:171–179
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29:15–21
Dong J, Chen W (2013) The role of autophagy in chloroplast degradation and chlorophagy in immune defenses during Pst DC3000 (AvrRps4) infection. PLoS ONE 8:e73091
El Kasmi F, Chung EH, Anderson RG, Li JY, Wan L, Eitas TK, Gao ZY, Dangl JL (2017) Signaling from the plasma-membrane localized plant immune receptor RPM1 requires self-association of the full-length protein. Proc Natl Acad Sci USA 114:E7385–E7394
Gao Z, Chung EH, Eitas TK, Dangl JL (2011) Plant intracellular innate immune receptor Resistance to Pseudomonas syringae pv. maculicola 1 (RPM1) is activated at, and functions on, the plasma membrane. Proc Natl Acad Sci USA 108:7619–7624
Grant MR, Jones JDG (2009) Hormone (Dis)harmony moulds plant health and disease. Science 324:750–752
Guo Y, Gan SS (2014) Translational researches on leaf senescence for enhancing plant productivity and quality. J Exp Bot 65:3901–3913
Ishida Y, Tsunashima M, Hiei Y, Komari T (2015) Wheat (Triticum aestivum L.) transformation using immature embryos. In: Wang K (ed) Agrobacterium protocols, 3rd edn, vol 1, pp 189–198
Jiang H, Li M, Liang N, Yan H, Wei Y, Xu X, Liu J, Xu Z, Chen F, Wu G (2007) Molecular cloning and function analysis of the stay green gene in rice. Plant J 52:197–209
Jiao BB, Wang JJ, Zhu XD, Zeng LJ, Li Q, He ZH (2012) A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice. Mol Plant 5:205–217
Kusaba M, Ito H, Morita R, Iida S, Sato Y, Fujimoto M, Kawasaki S, Tanaka R, Hirochika H, Nishimura M, Tanaka A (2007) Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19:1362–1375
Kwon SI, Park OK (2008) Autophagy in plants. J Plant Biol 51:313–320
Lee RH, Wang CH, Huang LT, Chen SCG (2001) Leaf senescence in rice plants: cloning and characterization of senescence up-regulated genes. J Exp Bot 52:1117–1121
Lee S, Masclaux-Daubresse C (2021) Current understanding of leaf senescence in rice. Int J Mol Sci 22
Li F, Vierstra RD (2012) Autophagy: a multifaceted intracellular system for bulk and selective recycling. Trends Plant Sci 17:526–537
Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760
Li M, Kim C (2022) Chloroplast ROS and stress signaling. Plant Commun 3:100264
Li Z, Zhang Y, Zou D, Zhao Y, Wang HL, Zhang Y, Xia X, Luo J, Guo H, Zhang Z (2020) LSD 3.0: a comprehensive resource for the leaf senescence research community. Nucleic Acids Res 48:D1069–D1075
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136
Liu LJ, Zhang YY, Tang SY, Zhao QZ, Zhang ZH, Zhang HW, Dong L, Guo HS, Xie Q (2010) An efficient system to detect protein ubiquitination by agroinfiltration in Nicotiana benthamiana. Plant J 61:893–903
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15
Mackey D, Holt BF, Wiig A, Dangl JL (2002) RIN4 interacts with Pseudomonas syringae type III effector molecules and is required for RPM1-mediated resistance in Arabidopsis. Cell 108:743–754
McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303
Morales F, Ancin M, Fakhet D, Gonzalez-Torralba J, Gamez AL, Seminario A, Soba D, Ben Mariem S, Garriga M, Aranjuelo I (2020) Photosynthetic metabolism under stressful growth conditions as a bases for crop breeding and yield improvement. Plants (Basel) 9
Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M (2009) Defect in non-yellow coloring 3, an alpha/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J 59:940–952
Murray MG, Thompson WF (1980) Rapid isolation of high molecular-weight plant DNA. Nucleic Acids Res 8:4321–4325
Qi H, Xia FN, Xiao S (2021) Autophagy in plants: Physiological roles and post-translational regulation. J Integr Plant Biol 63:161–179
Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5:278–282
Sobieszczuk-Nowicka E, Wrzesinski T, Bagniewska-Zadworna A, Kubala S, Rucinska-Sobkowiak R, Polcyn W, Misztal L, Mattoo AK (2018) Physio-genetic dissection of dark-induced leaf senescence and timing its reversal in Barley. Plant Physiol 178:654–671
Thomas H (2000) Five ways to stay green. J Exp Bot 329–337
Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 314:1298–1301
Wang J, Han M, Liu Y (2021) Diversity, structure and function of the coiled-coil domains of plant NLR immune receptors. J Integr Plant Biol 63:283–296
Wang P, Nolan TM, Yin Y, Bassham DC (2020) Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis. Autophagy 16:123–139
Wu XY, Kuai BK, Jia JZ, Jing HC (2012) Regulation of leaf senescence and crop genetic improvement. J Integr Plant Biol 54:936–952
Yin Z, Pascual C, Klionsky DJ (2016) Autophagy: machinery and regulation. Microb Cell 3:588–596
Yoshida S, Ito M, Nishida I, Watanabe A (2002) Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defence responses in Arabidopsis thaliana. Plant J 29:427–437
Zhao D, Derkx AP, Liu DC, Buchner P, Hawkesford MJ (2015) Overexpression of a NAC transcription factor delays leaf senescence and increases grain nitrogen concentration in wheat. Plant Biol (Stuttg) 17:904–913
Zhao L, Zhang W, Song Q, Xuan Y, Li K, Cheng L, Qiao H, Wang G, Zhou C (2020) A WRKY transcription factor, TaWRKY40-D, promotes leaf senescence associated with jasmonic acid and abscisic acid pathways in wheat. Plant Biol (Stuttg) 22:1072–1085
Acknowledgements
We thank Dr. Zhiyong Liu (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for providing the EMS-mutagenized population. We thank Drs. Huaizhi Zhang and Guanghao Guo (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences) for stripe rust resistance phenotyping at the adult plant stage.
Funding
This work was supported by National Natural Science Foundation of China (grant no. 32125030), National Key Research and Development Program of China (2020YFE0202300), and Pinduoduo-China Agricultural University Research Fund (PC2023A01003).
Author information
Authors and Affiliations
Contributions
Yingyin Yao and Jinkun Du conceived the project; Wenjia Zhang performed the experiments; Zhaoheng Zhang, Zihao Wang and Wanjun Song performed bioinformatics analysis; Qian Chen and Kai Yang provided technological assistance; Mingming Xin, Zhaorong Hu, Jie Liu, Huiru Peng, Jinsheng Lai, Weilong Guo, Zhongfu Ni, and Oixin Sun provided theoretical contributions to the project; Wenjia Zhang, Yingyin Yao and Jinkun Du contributed to article writing and revision.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Xiaoquan Qi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
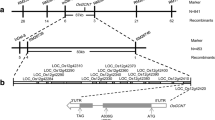
Fig. S1 Phenotypic and genetic analysis of F1 and F2 progenies. (a) Phenotypic of the WT, ylp and the F1 from cross between ylp mutant and WT at anthesis stage. Bar, 10 cm. (b) Phenotypic of the Baofeng104 (BF104), ylp and the F1 from cross between ylp and BF104 at anthesis stage. Bar, 10 cm. (c) The segregation ratio of F2 progeny derived from self-pollination of BF104 × ylp.
Fig. S2 Heatmap representing the transcriptional changes of the YLP fine localization region genes in ylp mutants relative to WT. Only four genes were expressed in the fine localization region of YLP. TraesCSC2D01G059100 and TraesCSC2D01G060500 were expressed in the leaves of seedling stage (a) and heading stage (b), while TraesCSC2D01G059400 was only expressed at seedling stage (a) and TraesCSC2D01G059300 was only expressed at heading stage (b). Heat map illustrating the FPKM-based expression patterns of genes in seedling stage (a) and heading stage (b) leaf of the WT and ylp. For each gene, the average value of FPKM values of three biological replicates. Red text indicates the expressed genes.
Fig. S3 Foliar tissues of the WT and the ylp mutants post-inoculated with the Pst races CYR34 were photographed at 20 dpi. Bar, 5 cm.
Fig. S4 Nicotiana benthamiana leaves were agro-infiltrated with constructs expressing heterozygous allele (co-expressed RPM1-H-GFP and RPM1-R-GFP), RPM1-R from WT and RPM1-H from ylp, respectively. Representative leaves were imaged 2, 3, and 4 d after agro-infiltration.
Fig. S5 Heatmap representing the transcriptional changes of plant-type hypersensitive response (HR) genes in ylp mutants relative to WT. Heat map illustrating the FPKM-based expression patterns of HR response gene in seedling stage leaf of the WT and ylp. For each gene, the average value of FPKM values of three biological replicates for each sample was normalized by R and reported as a heatmap.
Fig. S6 Heatmap representing the transcriptional changes of reactive oxygen species (ROS) responsive genes in ylp mutant relative to WT. Heat map illustrating the FPKM-based expression patterns of ROS responsive genes in seedling stage leaf of the WT and ylp. For each gene, the average value of FPKM values of three biological replicates for each sample was normalized by R and reported as a heatmap.
Fig. S7 Heatmap representing the transcriptional changes of pathogenesis-related (PR) defense response marker genes in ylp mutant relative to WT. Heatmap representing the transcriptional changes of pathogenesis-related (PR) defense response marker genes in ylp mutants relative to WT. Heat map illustrating the FPKM-based expression patterns of pathogenesis-related (PR) defense response marker genes in seedling stage leaf of the WT and ylp. For each gene, the average value of FPKM values of three biological replicates for each sample was normalized by R and reported as a heatmap.
Fig. S8 Subcellular localization of RPM1-R and its mutant form RPM1-H. Transient expression of 35S::TaRPM1-R-GFP, 35S::TaRPM1-H-GFP and 35S::PIP2-RFP (OD600 = 0.2) was performed in Nicotiana benthamiana leaves. Images were collected at the time 28 h after agrobacteria infiltration. 35S:PIP2-RFP (OD600 = 0.2) was used as a plasma membrane marker.
Below is the link to the electronic supplementary material.








Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, W., Zhang, Z., Chen, Q. et al. Mutation of a highly conserved amino acid in RPM1 causes leaf yellowing and premature senescence in wheat. Theor Appl Genet 136, 254 (2023). https://doi.org/10.1007/s00122-023-04499-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-023-04499-4