Abstract
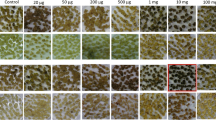
We report here the isolation of spectinomycin-resistant mutants in cultured cells of Medicago sativa line RegenSY-T2. Spectinomycin induces bleaching of cultured alfalfa cells due to inhibition of protein synthesis on the prokaryotic type 70S plastid ribosomes. Spontaneous mutants resistant to spectinomycin bleaching were identified by their ability to form green shoots on plant regeneration medium containing selective spectinomycin concentrations in the range of 25–50 mg/l. Sequencing of the plastid rrn16 gene revealed that spectinomycin resistance is due to mutations in a conserved stem structure of the 16S rRNA. Resistant plants transferred to the greenhouse developed normally and produced spectinomycin-resistant seed progeny. In light of their absence in soybean, a related leguminous plant, the isolation of spectinomycin-resistant mutants in M. sativa was unexpected. The new mutations are useful for the study of plastid inheritance, as demonstrated by detection of predominantly paternal plastid inheritance in the RegenSY-T2 × Szapko57 cross, and can be used as selective markers in plastid transformation vectors to obtain cisgenic plants.



Similar content being viewed by others
References
Austin S, Bingham ET, Mathews DE, Shahan MN, Will J, Burgess RR (1995) Production and field performance of transgenic alfalfa (Medicago sativa L.) expressing alpha-amylase and manganese-dependent lignin peroxidase. Euphytica 85:381–393
Azhagiri A, Maliga P (2007) Exceptional paternal inheritance of plastids in Arabidopsis suggests that low frequency leakage of plastid via pollen may be universal in plants. Plant J 52:817–823
Bingham ET, Hurley LY, Kaatz DM, Saunders JW (1975) Breeding alflafa which regenerates from callus tissue in culture. Crop Sci 15:719–721
Bock R (2001) Transgenic plastids in basic research and plant biotechnology. J Mol Biol 312:425–438
Brown DCW, Atanassov A (1985) Role of genetic background in somatic embryogenesis. Plant Cell Tiss Org Cult 4:111–122
Carrer H, Hockenberry TN, Svab Z, Maliga P (1993) Kanamycin resistance as a selectable marker for plastid transformation in tobacco. Mol Gen Genet 241:49–56
Chumley TW, Palmer JD, Mower JP, Fourcade HM, Calie PJ, Boore JL, Jansen RK (2006) The complete chloroplast genome sequence of Pelargonium × hortorum: organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol Biol Evol 23:2175–2190
Craig W, Lenzi P, Scotti N, De Palma M, Saggese P, Carbone V, McGrath Curran N, Magee AM, Medgyesy P, Kavanagh TA, Dix PJ, Grillo S, Cardi T (2008) Transplastomic tobacco plants expressing a fatty acid desaturase gene exhibit altered fatty acid profiles and improved cold tolerance. Transgenic Res 17:769–782
Deak M, Kiss GB, Koncz C, Dudits D (1986) Transformation of Medicago by Agrobacterium mediated gene transfer. Plant Cell Rep 5:97–100
Dufourmantel N, Pelissier B, Garcon F, Peltier G, Ferullo JM, Tissot G (2004) Generation of fertile transplastomic soybean. Plant Mol Biol 55:479–489
Fromm H, Edelman M, Aviv D, Galun E (1987) The molecular basis for rDNA-dependent spectinomycin resistance in Nicotiana chloroplasts. EMBO J 6:3233–3237
Golds T, Maliga P, Koop HU (1993) Stable plastid transformation in PEG-treated protoplasts of Nicotiana tabacum. Biotechnology 11:95–97
Hagemann R (2002) Milestones in plastid genetics of higher plants. Prog Bot 63:5–51
Huang FC, Klaus SMJ, Herz S, Zuo Z, Koop HU, Golds TJ (2002) Efficient plastid transformation in tobacco using the aphA-6 gene and kanamycin selection. Mol Genet Genomics 268:19–27
Kanamoto H, Yamashita A, Asao H, Okumura S, Takase H, Hattori M, Yokota A, Tomizawa K (2006) Efficient and stable transformation of Lactuca sativa L. cv. Cisco (lettuce) plastids. Transgenic Res 15:205–217
Kavanagh TA, O’Driscoll KM, McCabe PF, Dix PJ (1994) Mutations conferring lincomycin, spectinomycin, and streptomycin resistance in Solanum nigrum are located in three different chloroplast genes. Mol Gen Genet 242:675–680
Khoudi H, Laberge S, Ferullo JM, Bazin R, Darveau A, Castonguay Y, Allard G, Lemieux R, Vézina LP (1999) Production of a diagnostic monoclonal antibody in perennial alfalfa plants. Biotechnol Bioeng 64:135–143
Koop HU, Herz S, Golds TJ, Nickelsen J (2007) The genetic transformation of plastids. In: Bock R (ed) Cell and molecular biology of plastids. Springer, Berlin, pp 457–510
Lelivelt C, McCabe M, Newell C, de Snoo B, Van Dunn K, Birch-Machin I, Gray JC, Mills K, Nugent JM (2005) Plastid transformation in lettuce (Lactuca sativa L). Plant Mol Biol 58:763–774
Maliga P (2004) Plastid transformation in higher plants. Ann Rev Plant Biol 55:289–313
Maliga P, Bock R (2011) Plastid biotechnology: food, fuel and medicine for the 21st century. Plant Physiol 155:1501–1510
Masoud SA, Johnson LB, Sorensen EL (1990) High transmission of paternal plastid DNA in alfalfa plants demonstrated by restriction fragment polymorphic analysis. Theor Appl Genet 79:49–55
Mogensen HL (1996) The hows and whys of cytoplasmic inheritance in seed plants. Am J Bot 83:383–404
Reboud X, Zeyl C (1994) Organelle inheritance in plants. Heredity 72:132–140
Rosellini D, LaFayette PR, Barone P, Veronesi F, Parrott WA (2004) Kanamycin-resistant alfalfa has a point mutation in the 16S plastid rRNA. Plant Cell Rep 22:774–779
Ruf S, Hermann M, Berger IJ, Carrer H, Bock R (2001) Stable genetic transformation of tomato plastids: foreign protein expression in fruit. Nat Biotechnol 19:870–875
Ruf S, Karcher D, Bock R (2007) Determining the transgene containment level provided by chloroplast transformation. Proc Natl Acad Sci USA 104:6998–7002
Ruhlman T, Verma D, Samson N, Daniell H (2010) The role of heterologous chloroplast sequence elements in transgene integration and expression. Plant Physiol 152:2088–2104
Schouten HJ, Krens FA, Jacobsen E (2006) Cisgenic plants are similar to traditionally bred plants: international regulations for genetically modified organisms should be altered to exempt cisgenesis. EMBO Rep 7:750–753
Schumann CM, Hanckok JF (1989) Paternal inheritance of plastids in Medicago sativa. Theor Appl Genet 78:863–866
Shaver JM, Oldenburg DJ, Bendich AJ (2006) Changes in chloroplast DNA during development in tobacco, Medicago truncatula, pea, and maize. Planta 224:72–82
Skarjinskaia M, Svab Z, Maliga P (2003) Plastid transformation in Lesquerella fendleri, an oilseed Brassicacea. Transgenic Res 12:115–122
Smith SE (1989) Influence of parental genotype on plastid inheritance in Medicago sativa. J Hered 80:214–217
Smith SE, Bingham ET, Fulton RW (1986) Transmission of chlorophyll deficiencies in Medicago sativa. J Hered 77:35–38
Sourrouille C, Marquet-Blouin E, D’Aoust MA, Kiefer-Meyer MC, Seveno M, Pagny-Salehabadi S, Bardor M, Durambur G, Lerouge P, Vezina L, Gomord V (2008) Down-regulated expression of plant-specific glycoepitopes in alfalfa. Plant Biotechnol J 6:702–721
Staub JM, Maliga P (1992) Long regions of homologous DNA are incorporated into the tobacco plastid genome by transformation. Plant Cell 4:39–45
Staub JM, Maliga P (1993) Accumulation of D1 polypeptide in tobacco plastids is regulated via the untranslated region of the psbA mRNA. EMBO J 12:601–606
Svab Z, Maliga P (1991) Mutation proximal to the tRNA binding region of the Nicotiana plastid 16S rRNA confers resistance to spectinomycin. Mol Gen Genet 228:316–319
Svab Z, Maliga P (1993) High-frequency plastid transformation in tobacco by selection for a chimeric aadA gene. Proc Natl Acad Sci USA 90:913–917
Svab Z, Maliga P (2007) Exceptional transmission of plastids and mitochondria from the transplastomic pollen parent and its impact on transgene containment. Proc Natl Acad Sci USA 104:7003–7008
Svab Z, Hajdukiewicz P, Maliga P (1990) Stable transformation of plastids in higher plants. Proc Natl Acad Sci USA 87:8526–8530
Thyssen G, Svab Z, Maliga P (2012) Exceptional inheritance of plastids via pollen in Nicotiana sylvestris with no detectable paternal mitochondrial DNA in progeny. Plant J. doi:10.1111/j.1365-313X.2012.05057.x
Valkov VT, Gargano D, Manna C, Formisano G, Dix PJ, Gray JC, Scotti N, Cardi T (2011) High efficiency plastid transformation in potato and regulation of transgene expression in leaves and tubers by alternative 5′ and 3′ regulatory sequences. Transgenic Res 20:137–151
Wakasugi T, Tsudzuki T, Sugiura M (2001) The genomics of land plant chloroplasts: gene content and alteration of genomic information by RNA editing. Photosynth Res 70:107–118
Waltz E (2011) GM grass eludes outmoded USDA oversight. Nat Biotechnol 29:772–773
Wei Z, Liu Y, Lin C, Wang Y, Cai Q, Dong Y, Xing S (2011) Transformation of alfalfa chloroplasts and expression of green fluorescent protein in a forage crop. Biotechnol Lett 33:2487–2494
Zoschke R, Liere K, Borner T (2007) From seedling to mature plant: Arabidopsis plastidial genome copy number, RNA accumulation and transcription are differentially regulated during leaf development. Plant J 50:710–722
Acknowledgments
This research was supported by the HSRF Grant K-82037 and the Research and Development Fund of the Ministry of Agriculture, Hungary. We thank Ms. Ágnes Mihály and Ms. Magdolna Péli for skillful technical assistance. Research on alfalfa plastid inheritance in PM’s laboratory at Rutgers University is supported by the USDA National Institute of Food and Agriculture Biotechnology Risk Assessment Research Grant Program Award No. 2010-2716.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Havey.
Rights and permissions
About this article
Cite this article
Dudas, B., Jenes, B., Kiss, G.B. et al. Spectinomycin resistance mutations in the rrn16 gene are new plastid markers in Medicago sativa . Theor Appl Genet 125, 1517–1523 (2012). https://doi.org/10.1007/s00122-012-1930-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-012-1930-7