Abstract
The obligate biotrophic, soil-borne fungus Synchytrium endobioticum causes wart disease of potato (Solanum tuberosum), which is a serious problem for crop production in countries with moderate climates. S. endobioticum induces hypertrophic cell divisions in plant host tissues leading to the formation of tumor-like structures. Potato wart is a quarantine disease and chemical control is not possible. From 38 S. endobioticum pathotypes occurring in Europe, pathotypes 1, 2, 6 and 18 are the most relevant. Genetic resistance to wart is available but only few current potato varieties are resistant to all four pathotypes. The phenotypic evaluation of wart resistance is laborious, time-consuming and sometimes ambiguous, which makes breeding for resistance difficult. Molecular markers diagnostic for genes for resistance to S. endobioticum pathotypes 1, 2, 6 and 18 would greatly facilitate the selection of new, resistant cultivars. Two tetraploid half-sib families (266 individuals) segregating for resistance to S. endobioticum pathotypes 1, 2, 6 and 18 were produced by crossing a resistant genotype with two different susceptible ones. The families were scored for five different wart resistance phenotypes. The distribution of mean resistance scores was quantitative in both families. Resistance to pathotypes 2, 6 and 18 was correlated and independent from resistance to pathotype 1. DNA pools were constructed from the most resistant and most susceptible individuals and screened with genome wide simple sequence repeat (SSR), inverted simple sequence region (ISSR) and randomly amplified polymorphic DNA (RAPD) markers. Bulked segregant analysis identified three SSR markers that were linked to wart resistance loci (Sen). Sen1-XI on chromosome XI conferred partial resistance to pathotype 1, Sen18-IX on chromosome IX to pathotype 18 and Sen2/6/18-I on chromosome I to pathotypes 2,6 and 18. Additional genotyping with 191 single nucleotide polymorphism (SNP) markers confirmed the localization of the Sen loci. Thirty-three SNP markers linked to the Sen loci permitted the dissection of Sen alleles that increased or decreased resistance to wart. The alleles were inherited from both the resistant and susceptible parents.
Similar content being viewed by others
Introduction
Potato wart is a disease that is increasingly becoming a problem for potato production in Europe. The causal agent of the disease is the obligate biotrophic, soil-borne fungus Synchytrium endobioticum (Schilberszky) Percival, which infects tubers, stolons and stems and can cause yield losses up to 50–100% (Baayen et al. 2006; Melnik 1998).The typical disease symptom is the formation of wart, a cauliflower-like multicellular tissue varying in size from few millimetres to several centimetres that contains the growing fungus and its sporangia. Wart tissue consists of hypertrophic, tumor-like dividing cells, which surround numerous, thin-walled summer and thick-walled winter spores or sporangia (sori). The sori are able to survive in the soil for up to four decades (Laidlaw 1985). The spreading of sporangia occurs mainly through infected tubers or contaminated agronomic tools. Under favorable climatic conditions, the sporangia sporulate and infect meristematic tissues throughout the vegetative period of potato plants (Stachewicz and Enzian 1998a, b). Therefore, the pathogen represents a long-term soil contamination problem, particularly in areas with moderate temperatures (8–10°C) and high soil humidity (50–80%). In Europe, the Ukraine and Turkey, 38 pathotypes of S. endobioticum have been identified so far (Baayen et al. 2006; Çakır et al. 2009). Pathotype 1 is known since long time and is considered as the “common pathotype”. More recently, new pathotypes were discovered that overcome the resistance to pathotype 1. Meanwhile, S. endobioticum pathotypes 2, 6 and 18 together with pathotype 1 represent the most widely distributed and aggressive forms of the fungus (Stachewicz 2002).
Chemical control of S. endobioticum is not possible. The only strategies to confine the disease are strict quarantine and phytosanitary measures on the one hand, and the cultivation of resistant cultivars on the other. Infected plants have to be destroyed and fields infested with S. endobioticum are not allowed to be planted with potatoes for at least 20 years. Safety zones are only allowed to be planted with cultivars resistant to the detected pathotype. Besides reduction of crop yield and quality, the main economic losses result from the phytosanitary measures, which prevent further potato cultivation on infested fields. Before World War II, wart was one of the most important potato diseases. The introduction of quarantine measures and of varieties resistant to S. endobioticum pathotype 1 were successful in controlling the disease. However, only 4% of the current German varieties are resistant to all four pathotypes 1, 2, 6 and 18, and these are of limited commercial importance as they often lack the agronomic qualities of modern high yielding varieties, which cover most of the potato cultivated area. The breeding of new varieties is therefore necessary, which combine wart resistance to pathotypes 1, 2, 6 and 18 with good quality traits. The screening for resistance to S. endobioticum (Langerfeld and Stachewicz 1994) is labor-intensive, time-consuming and sometimes ambiguous, as the pathogen causes disease symptoms with variable phenotypes. Resistance testing requires a large number of tubers due to the need for replicated inoculations. This prevents phenotypic selection for resistance early in the breeding process. Molecular markers diagnostic for resistance to different wart pathotypes would greatly facilitate the selection of resistant cultivars. Ideally, such markers are in linkage disequilibrium with or even residing within the resistance gene (Sattarzadeh et al. 2006).
Although resistance to wart has been one of the first plant traits subjected to Mendelian genetic analysis (Salaman and Lesley 1923), the genetics of potato resistance to S. endobioticum is still poorly understood. The molecular basis of wart formation and resistance to it is completely unknown. Several genetic models have been proposed to explain the phenotypic segregation of wart resistance. Observed segregation ratios of wart resistant and susceptible plants and the observation of wart-resistant plants among the F1 progeny of crosses between susceptible parents led to the hypothesis that combinations of two or more genes are required to express the resistance phenotype (Black 1935; Ross 1986; Salaman and Lesley 1923), or suppressor genes are involved (Salaman and Lesley 1923). Dominant genes in combination with other genes of minor or inhibitory effect have been suggested to control resistance to S. endobioticum (Lunden and Jørstad 1934; Maris 1973). Except Maris (1973), these early genetic studies were performed with tetraploid genotypes having tetrasomic inheritance but using models based on the assumption of disomic inheritance. In fact, with the help of molecular markers two dominant genes for resistance have been identified and mapped in experimental, diploid mapping populations. The Sen1 gene confers resistance to S. endobioticum pathotype 1 and maps on chromosome XI (Hehl et al. 1999), in a region of the potato genome that also contains a number of resistance genes against other pathogens (Gebhardt and Valkonen 2001). The DNA polymorphism that was diagnostic for Sen1 in the diploid parent used for mapping the Sen1 locus was not detectable in tetraploid breeding materials (unpublished observations). Brugmans et al. (2006) identified a second, independent locus for resistance to pathotype 1 on chromosome IV, named Sen1-4. The genomic locations of genes for resistance to other pathotypes are unknown. When dominant genes for resistance to various pathogens including Sen1 were pyramidized by marker-assisted selection, resistance against pathotypes 2 and 6 was found, which seemed to segregate independent of Sen1 (Gebhardt et al. 2006).
DNA-based molecular markers have made feasible the construction of precise genetic linkage maps in many eukaryotic organisms. The molecular basis of all DNA-based markers are point mutations (single nucleotide polymorphism, SNP), insertion–deletions or inversions of DNA fragments leading to molecular differences between the genomes of individuals of a given species. The technology to detect DNA polymorphisms in eukaryotes has undergone a rapid development over the last three decades since the introduction of restriction fragment length polymorphism (RFLP) analysis (Botstein et al. 1980), particularly with the advent of the polymerase chain reaction (PCR) (Mullis and Faloona 1987). Molecular linkage maps of potato have been constructed based on RFLP, amplified fragment length polymorphism (AFLP) (Vos et al. 1995), simple sequence repeat (SSR) (Hamada et al. 1982; Tautz and Renz 1984), inverted simple sequence regions (ISSR) (Tsumura et al. 1996), randomly amplified polymorphic DNA (RAPD) (Williams et al. 1990) and other PCR-based markers (Gebhardt 2007; Ghislain et al. 2009). The potato molecular maps have provided the framework for locating genes for monogenic and polygenic resistance to various pathogens on the twelve potato chromosomes (Gebhardt and Valkonen 2001; Simko et al. 2007) [see also the SOLanaceae function map for resistance at http://www.gabipd.org/database/maps.shtml in the Potato Maps and More (PoMaMo) database (Meyer et al. 2005)].
With the aim first, to better understand the genetic basis of the interaction of potato with different pathotypes of S. endobioticum and secondly, to identify DNA-markers closely linked to wart resistance genes in tetraploid potato, we performed a genetic and phenotypic analysis of wart resistance to S. endobioticum pathotypes 1, 2, 6 and 18 in two tetraploid mapping populations. Based on SSR, ISSR, RAPD and SNP markers we identified novel loci with multiple alleles for resistance to pathotypes 2, 6 and 18 and confirmed the Sen1 locus on chromosome XI.
Materials and methods
Plant material
Two tetraploid half-sib families were used for mapping. Crosses and tuber production were performed by the potato breeding companies Böhm-Nordkartoffel Agrarproduktion (BNA) and SaKa Pflanzenzucht (SaKa). The parent Pr was resistant to S. endobioticum pathotypes 1, 2, 6 and 18 (with mean scores of P1 = 2.0, P2 = 2.0, P6 = 2.0 and P18 = 2.1). Pr was crossed with two different susceptible clones Ps-SaKa (mean scores of P1 = 3.4, P2 = 4.8, P6 = 4.6 and P18 = 4.7) and Ps-BNA (mean scores of P1 = 4.6 and P6 = 5.0, P2 and P18 were not tested). The two F1 families, named BNA1 and SaKa1, consisted of 141 and 125 genotypes, respectively. The families were field propagated under the phytosanitary regimes used for seed tuber production.
Test for wart resistance
Five tubers in a first screen, and 20–40 tubers per genotype and pathotype in a second screen were tested for resistance to the four S endobioticum pathotypes 1, 2, 6 and 18 using a modified Glynne-Lemmerzahl method (Glynne 1925; Lemmerzahl 1930) described by (Langerfeld and Stachewicz 1994). Eye fields (3 × 3 cm tuber tissue surrounding a tuber eye) cut out from tubers with sprouts 1–2 mm in length were ringed with warm vaseline, using a syringe without needle. The ring was filled with distilled water and the sprout was inoculated by applying 3–4 weeks old wart tissue of the corresponding pathotype. After 48-h incubation at 10°C, the wart tissue was removed and the eye fields were treated with pencycoron in order to prevent infections with Rhizoctonia solani. The infected sprouts were covered with a moist, sterile soil/peat mixture of 2 cm thickness and incubated at 15–17°C at high humidity. The cultivars Tomensa, Combi, Sorka, Saphira, Dèsirèe, Miriam, Sissi, Karolin and Ulme were used as differentials for assessing the pathotype specificity of the wart inoculum. The scoring of the disease symptoms was done by examining each tuber sprout under a stereo microscope 25–28 days post-inoculation. Disease symptoms were scored from 1 to 5, 1 being most resistant and 5 most susceptible (Fig. 1) (Stachewicz et al. 2005). Mean scores were calculated from the individual scores of all infected tubers according to M = [a + 2b + 3c + 4d + 5e]/n, where a, b, c, d and e are the number of tubers scored with 1, 2, 3, 4 and 5, respectively, and n is the total number of scored tubers.
Phenotypes of the interaction between potato and Synchytrium endobioticum. a Extremely resistant: early defense necrosis, no sori detectable (score 1 = resistance group R1); b resistant: late defense necrosis, larger necrotic areas, sori immature or necrotic (score 2 = R1); c weakly resistant: very late defense necrosis, up to five non-necrotic sori per sprout (score 3 = R2); d slightly susceptible: scattered infections, sprout can be malformed (score 4 = S1); e extremely susceptible, predominant tumor formation, high number of non-necrotic sori (score 5 = S2)
Resistance was assessed qualitatively by classifying a genotype as resistant when all tubers tested showed an R1 or R2 type of interaction and as susceptible when one or more tubers showed the R3, S1 or S2 phenotype (Fig. 1).
Construction of pools for bulked segregant analysis (BSA)
Based on the evaluation for wart resistance of the 141 genotypes of the BNA1 family, individuals were selected to construct resistant and susceptible pools. Three pools were constructed based on the qualitative classification of individuals as resistant or susceptible. Pool-r1 was composed of 10 individuals most resistant to S. endobioticum pathotype 1, Pool-r1,2,6,18 consisted of eight individuals most resistant to pathotypes 1, 2, 6, 18 and Pool-s included ten individuals susceptible to all four pathotypes. Eight further pools, one resistant and one susceptible to each of the four pathotypes were constructed based on the phenotypic mean values. The extreme resistant pools P-ER1, P-ER2, P-ER6 and P-ER18 each contained five individuals with the lowest mean scores for resistance to pathotype 1, 2, 6 and 18, respectively. The extreme susceptible pools P-ES1, P-ES2, P-ES6 and P-ES18 were composed of five individuals each that had the highest mean scores for resistance to pathotype 1, 2, 6 and 18, respectively. The eleven pools were constructed by mixing equal amounts of genomic DNA extracted from the selected individuals.
Plant genomic DNA isolation
Total genomic DNA was isolated from freeze-dried leaf tissue (Bormann et al. 2004) using DNeasy Plant DNA Isolation Kit (Qiagen, Hilden, Germany) and a BioRobot 9600 (Qiagen) in a 96-well format, following the supplier’s protocols. The DNA concentration was measured with a NanoDrop® ND-1000 spectrophotometer V3 2.0 (Wilmington, USA). The DNA quality was examined on standard ethidium bromide containing agarose gels and by performing control PCRs using ubiquitin-specific primers UBQf (gaccatcactcttgaggttgag) and UBQr (aatggtgtctgagtctgagctctcgac) at an annealing temperature of 58°C, which amplified a 300-bp DNA fragment.
PCR analysis
Amplicons were generated from 50 ng genomic DNA template in a total volume of 25 μL buffer (20 mM Tris–HCl, pH 8.4, 1.5 mM MgCl2, 50 mM KCl), including 0.25 μM of each primer, 0.2 mM dNTPs and 0.2 Units Taq DNA polymerase (Invitrogen Life Technologies, Freiburg, Germany). Standard cycling conditions were 3 min initial denaturation at 94°C, followed by 39 cycles of 1 min denaturation at 94°C, 1 min annealing at the appropriate temperature, and 1 min extension at 72°C. Reactions were finished by 10 min incubation at 72°C. PCR products were size separated by agarose gel electrophoresis and visualized by ethidium bromide staining.
SSR, ISSR and RAPD markers
Simple sequence repeat markers were PCR amplified from genomic DNA using primers and conditions reported by (Feingold et al. 2005; Ghislain et al. 2009; Milbourne et al. 1998). SSR alleles were size separated on Spreadex gels (Elchrom Scientific, CH-6330 Cham, Switzerland) according to the supplier’s instructions. Polymorphic SSR alleles were scored as present (1) or absent (0). For RAPD analysis the OPERON 10-mer oligonucleotides (Qiagen) were used as primer at an annealing temperature of 35°C. PCR conditions were as described by (Williams et al. 1990) and (Ballvora et al. 1995). ISSR analysis was performed as described (Szajko et al. 2008).
Development of markers based on the potato genome sequence information
Marker sequences (www.gabipd.org/projects/Pomamo/) were used for BLAST analysis against the first draft version of the Solanum phureja genome sequence (http://www.potatogenome.net) in order to identify matching scaffolds (SC). The scaffold SC176 contained the DNA-sequence of the primers for SSR marker STM2030, SC15 matched to the sequences of both markers GP194 and GP124, SC202 matched to marker GP129, SC44 to the markers GP125, GP259 and GP163, and SC461 contained the sequence of BA30p15t7. Based on the sequence information of the scaffolds oligonucleotides were designed (supplementary Table 1) in order to amplify by PCR the corresponding genomic region.
SNP analysis
Amplicons were generated with locus-specific primers (supplementary Table 1) from approximately 50 ng genomic DNA of the tetraploid individuals of the BNA1 and SaKa1 families and the parents Pr and Ps-SaKa. The parent Ps-BNA was not available for genotyping. The amplicons were purified with ExoSAP-IT (USB, Cleveland, USA) and custom sequenced at the core facility for DNA analysis of the Max Planck Institute for Plant Breeding Research. The Sanger dideoxy chain-termination sequencing method was employed using an ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit and an ABI PRISM 3730 automated DNA sequencer (Applied Biosystems, Weiterstadt, Germany). SNPs were detected by sequence alignments and visual examination of the sequences for overlapping base-calling peaks. The bi-allelic SNPs were assigned in each tetraploid individual to one of five allelic states (two homozygous and three heterozygous). The SNP allele dosage in heterozygous individuals (1:3, 2:2 or 3:1) was estimated from the height ratio of the overlapping base-calling peaks using the data acquisition and analysis software DAX (Van Mierlo Software Consultancy, Eindhoven, The Netherlands). Insertion/deletion polymorphisms (indels) were detected in the sequence trace files of the amplicons by sequence overlaps starting at specific nucleotide positions.
Statistic analysis
The polymorphic fragments generated with SSR primers in the whole mapping populations were scored as absent (0) or present (1). The allele dosage of the SSR markers STM2030, STM3023b and StI046 was not scorable. The five allelic states of each bi-allelic SNP marker present in a tetraploid individual were scored with 0 and 4 for the two homozygous states and with 1, 2 or 3 for the heterozygous states (1:3, 2:2, 3:1). Kruskal–Wallis and analysis of variance (ANOVA) tests for linkage of segregating SSR and SNP alleles with the phenotypic resistance data were performed using the software SPSS 10.0 (SPSS GmbH, München, Germany). Both tests gave essentially the same results. A marker was considered to be linked with a resistance locus at P ≤ 0.05. Principal component analysis was done using Statistica (StatSoft (Europe) GmbH, Hamburg, Germany).
Results
Wart resistance assessment
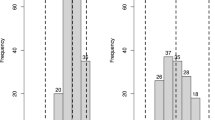
Nearly 16,000 tubers of 266 genotypes of two tetraploid half-sib families (BNA1 and SaKa1) were evaluated for resistance to S. endobioticum pathotypes 1, 2, 6 and 18 by assigning them to one of five interaction phenotypes (Fig. 1). Few genotypes from both families were classified as extremely resistant (mean score 2 or less, Fig. 1a, b) to any single pathotype, mainly for pathotype 1, but none of them was fully resistant (score R1) to all four pathotypes. Four genotypes were extremely susceptible (score 5, Fig. 1e) to pathotype 18 and one of them also to pathotype 6. The frequency distribution of the mean scores showed that resistance to all tested S. endobioticum pathotypes segregated as a quantitative trait in both families (Fig. 2). Whereas the distribution of resistance to pathotype 1 (Fig. 2a, e) was skewed towards resistance in both families (mean < 3) (χ 2 = 41.6; P < 0.0005 for BNA1 and χ 2 = 22.1; P < 0.0005 for SaKa1), the distribution of resistance to pathotypes 2 (χ 2 = 6.5; P < 0.05), 6 (χ 2 = 9.3; P < 0.005) and 18 (χ 2 = 10.9; P < 0.0025) was skewed towards susceptibility (mean > 3) in the SaKa1 family but only for pathotype 18 (χ 2 = 12.9; P < 0.001) in the BNA1 family. Although the wart-resistant parent was the same in both families, the distribution of resistant phenotypes in the progeny varied between the two families, indicating that the susceptible parents, which differed between the BNA1 and SaKa1 family, influenced the phenotypic expression of resistance to wart. Principal component analysis revealed that resistance to pathotypes 2, 6 and 18 was strongly correlated with each other but separated from resistance to pathotype 1 (Fig. 3).
Frequency distributions of the mean scores for resistance to S. endobioticum pathotypes. a–d Histograms of resistance to pathotypes 1, 2, 6 and 18, respectively, in the BNA1 family; e–h histograms of resistance to pathotypes 1, 2, 6 and 18, respectively, in the SaKa1 family. The resistance scores of the resistant (Pr) and susceptible (Ps) parents of BNA1 and SaKa1 are indicated by arrows. Data for resistance to pathotypes 2 and 6 were not available for the parent Ps of the BNA family
Identification of loci for resistance to S. endobioticum
Eleven DNA bulks were constructed based on the most resistant and most susceptible genotypes in the BNA1 family and screened with 420 RAPD, 96 ISSR and 96 SSR markers. RAPD and ISSR primers generated between one and seven DNA fragments with an average of three to five fragments per primer. The SSR primers amplified between three and five fragments. Twenty-two RAPD and seven ISSR primers detected reproducibly qualitative or quantitative polymorphisms between the pools. After re-amplification in the individual pool members, none of these polymorphic fragments had a significantly different frequency in resistant versus susceptible pools. Seventeen of 96 SSR primers amplified at least one fragment polymorphic between resistant and susceptible pools. Fourteen of those fragments still showed differences after re-amplification in the individual pool members and were used to genotype the whole BNA1 and SaKa1 families. ANOVA using the mean resistance scores and the marker data revealed three SSR markers that were linked with genes for resistance to S. endobioticum. These three SSR markers had been polymorphic in both pool types, the qualitative as well as the quantitative one (see “Materials and methods”). One allele of the SSR marker STM3023b, which maps to a distal part of the long arm of chromosome IX (Pajerowska-Mukhtar et al. 2009) showed significant linkage to a locus conferring partial resistance to S. endobioticum pathotype 18 in both the BNA1 (P = 0.002) and SaKa1 (P = 0.001) family. The allele STM3023b_1 descended from Pr (Fig. 4a). Marker StI046 maps to a distal part of chromosome XI (Feingold et al. 2005). The allele StI046_1 (Fig. 4b) segregating.in the BNA1 family was linked (P = 0.008) with partial resistance to S. endobioticum pathotype 1. StI046_1 descended from the susceptible parent of the BNA1 family. The StI046_1 allele could not be scored in the SaKa1 family. The third SSR marker STM2030 is located on potato chromosome I (Milbourne et al. 1998). The allele STM2030_1 (double fragment in Fig. 4c) was linked with partial resistance to S. endobioticum pathotypes 2, 6 and 18 (P < 0.001), indicating the presence of a locus on chromosome I, which confers resistance to all three pathotypes (Fig. 4c).
Amplification patterns of the SSR markers STM3023b (a), StI046 (b) and STM2030 (c) linked to loci for resistance to S. endobioticum (Sen). The PCR conditions for all the three markers were: 35 cycles at 93°C for 30 s, 55°C for 45 s, 72°C for 1.5 min and a final elongation of 10 min at 72°C. The PCR products were separated on a 600—Elchrom-gel for 60 min (a), a 600—Elchrom-Gel for 90 min (b) and a 300—Elchrom-Gel for 120 min (c), at 120 volt, and visualized under UV after staining with SYBR Gold Nucleic Acid Gel Staining (Invitrogen, Carlsbad, CA, USA). Arrows on the left of each panel mark the alleles STM3023b_1 (a), StI046_1 (b) and STM2030_1 (c); the allele size in base pairs also shown on the left was estimated relative to the size standards indicated by arrows on the right. The lanes labeled ‘Pool_s, Pool-r1, Pool-r1,2,6,18, P-ER1, P-ES1, P-ER6 and P-ES6’ show the amplification products obtained with the corresponding DNA pools as described in “Materials and methods”. Pr and Ps are the resistant and susceptible parents of the SaKa1 family (a and c) and of the BNA1 family (b). The lanes labeled 1–6 show the amplification products of six randomly selected genotypes from the SaKa1 family (a and c) and the BNA1 family (b)
Genetic dissection of the chromosomal regions harboring wart resistance loci
To confirm the map positions and linkage of STM2030, STM3023b and StI046 with the wart resistance loci and to further dissect their allelic structure, both families were genotyped by amplicon sequencing for SNP markers at 13 loci in a 30 cM interval on chromosome I (CP19–GP258, http://www.gabipd.org/database/maps.shtml), at 4 loci covering approximately 40 cM on chromosome IX (GP129-St_At3g24010) and at 5 loci in a 3 cM interval on chromosome XI (GP163–GP259) (supplementary Table 1). The three chromosomal regions included the SSR loci STM2030, STM3023b and StI046, respectively. The 22 loci included five new markers developed from scaffolds of the draft genome sequence of S. phureja (http://www.potatogenome.net) selected with markers of known position on chromosomes I, IX and XI. In all 3,7-Mbp sequence information was generated from the 266 individuals. Hundred and ninety-one SNP markers were identified and scored in the amplicons, with an average of one SNP every 73 nucleotides (supplementary Table 1, sequences with SNP positions in supplementary material). When individually tested for linkage with resistance to S. endobioticum pathotypes 1, 2, 6 and 18, thirty-three SNPs were significantly linked with resistance to one or more S. endobioticum pathotypes (Table 1). Besides SSR marker STM2030, SNPs at the loci GP192, GP124, GP194 and SC176 on chromosome I showed linkage with resistance to pathotypes 2, 6 and 18. In the SaKa1 family, linkage with resistance to pathotype 1 was also detected. The marker loci cover a 9-cM map segment on the long arm of chromosome I (http://www.gabipd.org/database/maps.shtml). On chromosome IX, in addition to SSR marker STM3023b, SNPs at the loci GP129 and GP101 were primarily linked with resistance to pathotype 18. The three markers are located in a 16-cM map segment on the long arm of chromosome IX. Finally, SNPs at the loci St_At5g16710 (the potato ortholog of the Arabidopsis gene At5g16710), GP125 and GP259 were primarily linked with resistance to pathotype 1, in addition to the SSR marker StI046. The three closely linked markers (1 cM) map to a distal region on the long arm of chromosome XI, the same genomic region where the Sen1 locus conferring resistance to S. endobioticum pathotype 1 has been mapped previously (Hehl et al. 1999).
Single SNP alleles, which either co-segregated or were tightly linked in the BNA1 and SaKa1 families, were grouped into haplotypes (Table 1). The dosage of SNP haplotypes and alleles in the three parents was obtained either directly from the genotypic classes observed in Pr and Ps-SaKa or, in the case of Ps-BNA (not available for genotyping), was deduced indirectly from the genotypic classes observed in the BNA1 progeny. Three, three and eight SNP alleles and haplotypes were identified on chromosome I, IX and XI, respectively, which were linked either with positive (increasing resistance) or negative (decreasing resistance) alleles at the loci Sen2/6/18-I, Sen18-IX and Sen1-XI, respectively (Table 2). Positive and negative alleles were inherited from all three parents. Seven alleles showed the same positive or negative effect in both families, whereas one allele (Sen18-b) showed an opposite effect, positive in the SaKa1 and negative in the BNA1 family. Two alleles were significant in the SaKa1 family but not in the BNA1 family and two alleles were present in the PS-BNA but absent in PS-SaKa family.
Discussion
Nearly 16,000 sprouting tubers of two half-sib families comprising 266 tetraploid genotypes were inoculated with four S. endobioticum pathotypes and evaluated for five phenotypic interaction types between the fungus and its host. To the best of our knowledge, this is the first report on a joint evaluation of wart resistance to four S. endobioticum pathotypes in potato segregating populations. In assessing a tetraploid individual’s true resistance to wart, we experienced similar difficulties, which were encountered by earlier genetical research aimed at solving the puzzle of the inheritance of resistance to wart (Black 1935; Lunden and Jørstad 1934; Maris 1973; Salaman and Lesley 1923). A clear cut resistance phenotype (all tuber sprouts scored as R1 or R2, Fig. 1) and on the contrary full susceptibility (all tuber sprouts scored as S1 or S2) were observed, but only a minority of genotypes showed these extreme phenotypes. In many cases, tubers of the same genotype showed variable interaction phenotypes over the whole range from R1 (fast response with small necrotic lesions formed) to S2 (prolific wart tissue formed). Multiple, interacting resistance factors, incomplete penetrance of a present R gene, variable developmental states of the inoculated tissues and variability of the inoculum can be some reasons for this phenotypic variation. Due to the ambiguous phenotypes, it was not possible to deduce Mendelian segregation ratios from the phenotypic data. Instead, resistance was treated as quantitative trait for detecting linkage with DNA markers. Considering resistance to pathotype 1, the phenotypic distributions observed in the two tetraploid families contrasted the ones obtained earlier in diploid mapping populations (Brugmans et al. 2006; Hehl et al. 1999), where resistance segregated as a monogenic trait. Despite the phenotypic differences, Sen1-XI was mapped to the same genomic region as Sen1, suggesting that allelic variants of the same gene or members of a clustered gene family might encode quantitative and qualitative resistance to S. endobioticum pathotype 1.
Three SSR markers linked with wart resistance loci were identified by performing bulked segregant analysis (BSA) (Michelmore et al. 1991) in the BNA1 family. The two pooling strategies, one based on qualitative, the other one on quantitative assessment of resistance, proved similarly effective in identifying these markers. Pool construction was limited by the small number of individuals available with extreme phenotypes. The small pool sizes with only five individuals in the eight quantitative pools might have been one reason for the large number of false-positive markers found, which showed initially polymorphism between pools but later on no linkage with resistance. The SSR markers linked to Sen loci are located on potato chromosomes I, IX and XI. The three Sen loci tagged by the SSR markers were confirmed in both the BNA1 and SaKa1 family with additional SNP markers. The locus Sen2/6/18-I on chromosome I affected resistance mainly to pathotypes 2, 6 and 18, whereas the locus Sen1-XI on chromosome XI affected predominantly resistance to pathotype 1. This is consistent with the observed phenotypic correlation between resistance to pathotypes 2, 6 and 18 and independence of resistance to pathotype 1. The molecular basis of the Sen2/6/18-I locus can be a single gene that simultaneously affects resistance to pathotype 2, 6 and 18. Alternatively, several closely linked genes might confer pathotype-specific resistance. The third locus, Sen18-IX on chromosome IX, had a small effect on resistance primarily to pathotype 18. The five marker loci linked to Sen2/6/18-I (STM2030, SC176, GP192, GP124, GP194) tag a region on potato chromosome I, where no other genes for pathogen resistance have been identified so far. In contrast, the marker loci linked to Sen18-IX (GP129, GP101, STM3023b) and particularly Sen1-XI (GP125, GP259, StI046, St_At5g16710) map in resistance hot spots on potato chromosomes IX and XI, respectively, where genes for qualitative and quantitative resistance to various pathogens have been found previously (Gebhardt and Valkonen 2001; Simko et al. 2007) (see also the Solanaceae function map for pathogen resistance at http://www.gabipd.org/database/maps.shtml). At the molecular level, the distal part of the long arm of chromosome XI harbors families of nucleotide-binding (NB), leucine-rich repeat (LRR)-type genes, which are syntenic and homologous to the tobacco N gene for resistance to Tobacco Mosaic Virus (TMV) (Hehl et al. 1999; Vidal et al. 2002; Whitham et al. 1994). Members of these NB-LRR-type gene families are good candidates for being the molecular basis of the Sen1 loci.
Resistance to S. endobioticum is controlled by a minimum of three loci in the two analyzed families. Additional loci may have escaped detection for several reasons. Although the DNA pools were screened for polymorphisms at approximately 1,600–2,600 marker loci, this number might have been insufficient in saturating with markers all 48 chromosomes of the tetraploid potato genome. Other major resistance loci, for example Sen1-4 (Brugmans et al. 2006) could have been present in the BNA1 and SaKa1 families but escaped detection by BSA. Minor or modifier loci potentially segregating in the BNA1 and SaKa1 families are unlikely to be detected by BSA. To identify putative additional Sen loci, extensive genotyping of the whole populations and quantitative trait locus (QTL) analysis are required.
The scoring of SNPs including the SNP allele dosage at marker loci linked with wart resistance made possible a detailed analysis of allele composition at the Sen loci. In tetraploid species with tetrasomic inheritance such as potato, scoring an allele as present or absent (as done for the SSR markers) is equivalent to scoring the allele dosage from 0 to 4 only for the genetic model Aaaa × aaaa. In all other models, the frequency of the homozygous recessive genotype aaaa (allele absent) decreases from 25 (Aaaa × Aaaa) to 16.6% (AAaa × aaaa), 8.3% (AAaa × Aaaa), 2.8 (AAaa × AAaa) to 0% (for example, AAAa × aaaa). The power to detect a phenotypic difference between genotypic classes with the allele present (A +) and absent (aaaa) decreases to zero accordingly. Scoring the SNP allele dosage followed by test statistics using the genotypic classes AAAA, AAAa, AAaa, Aaaa and aaaa as grouping variable was therefore necessary for detecting Sen alleles such as Sen1-b and Sen1-g (Table 2).
Further genetic dissection of the three Sen loci by SNP genotyping revealed at each locus the presence of multiple alleles with positive as well as negative effects on wart resistance, which were inherited from both the resistant and the susceptible parents. These alleles are either functional variants of a single gene or of physically linked gene families, for example of the NB-LRR-type. The latter model seems more appropriate for the Sen1-XI locus, because allele number and parental dosage recorded here are difficult to reconcile with a single gene model with allows maximal four alleles. Resistance and susceptibility alleles both inherited from the susceptible parents explain the observation that the phenotypic distributions of wart resistance differed between the BNA1 and Saka1 families, despite the common resistant parent. The presence of susceptibility alleles in the resistant parent implicates dominance of resistance in this genotype, whereas the presence of resistance alleles in the susceptible parents implicates dominance of susceptibility in other genotypes. Taking these observations together, it appears that the resistance phenotype to S. endobioticum is the result of the composition and interaction of several alleles at three loci minimum, and therefore depends on the genetic background. In tetraploid individuals, the number of alleles is doubled and their possible combinations quadrupled compared to diploids, which might explain why the resistance phenotype appears quantitative in the former and qualitative in the latter, at least for pathotype 1. The genetic structure of resistance to wart as revealed in this study by DNA-markers does not contradict, in principal, the early classical genetic studies (Black 1935; Lunden and Jørstad 1934; Salaman and Lesley 1923) when we take into consideration that the segregation of four alleles at a single locus under the assumption of tetrasomic inheritance is equivalent to the segregation of two alleles at two independent loci assuming disomic inheritance. It also can explain the reported emergence of wart-resistant genotypes in progeny of susceptible parents (Black 1935; Salaman and Lesley 1923), the proposition of modifying and inhibitory genes (Lunden and Jørstad 1934; Maris 1973) and the discrepancies between the genetic models proposed by the different researchers. The genetic structure of resistance to wart in tetraploid germplasm has implications for marker-assisted selection. Allele combinations rather than single alleles should be selected, in resistant as well as in susceptible parental genotypes and their descendants. The SNP and SSR markers described in this paper provide the basis for molecular screening of potato germplasm of various origins, which might lead to novel, promising cross combinations targeted at the improvement of resistance to multiple pathotypes of S. endobioticum.
The diagnostic power of the DNA markers indentified in this study in genetic material other than the BNA1 and SaKa1 families is not clear at present. Analyzing nearly 200 SNP markers in the map segments that harbored the SSR markers did not permit a precise localization of the wart-resistance loci based on gradients of the quantitative effects across linked marker loci. Several Centimorgan might still separate the Sen loci from the most closely linked markers. Recombination is the most likely reason for the opposite effect of the SNP marker tagging allele Sen18-b that was observed in the two families. Association genetics (Gupta et al. 2005) provides the means to test whether some of the markers described in this paper are in linkage disequilibrium with Sen alleles present in tetraploid varieties and breeding clones. According to Ross (Ross 1986), resistance to S. endobioticum in cultivated potato has been introgressed from several sources, among others from the wild species S. acaule. Variety passport data are incomplete with respect to wart resistance or susceptibility, due to the variability of the evaluation methods used in different countries. This prevents a straightforward association test using passport data as was possible, for example, for resistance to the root cyst nematode Globodera pallida (Sattarzadeh et al. 2006). The marker allele STM2030_1 (linked to the wart resistance allele Sen2/6/18-a) was present in two of eight varieties known to be resistant to pathotypes 1, 2, 6 and 18 but was also found in some susceptible varieties (unpublished results). Further genetic studies are needed before diagnostic markers for wart resistance in a broader genetic background become available.
References
Baayen R, Cochius G, Hendriks H, Meffert J, Bakker J, Bekker M, van den Boogert P, Stachewicz H, van Leeuwen G (2006) History of potato wart disease in Europe—a proposal for harmonisation in defining pathotypes. Eur J Plant Pathol 116:21–31
Ballvora A, Hesselbach J, Niewohner J, Leister D, Salamini F, Gebhardt C (1995) Marker enrichment and high-resolution map of the segment of potato chromosome VII harbouring the nematode resistance gene Gro1. Mol Gen Genet 249:82–90
Black W (1935) Studies on the inheritance of resistance to wart disease (Synchytrium endobioticum (Schilb.) Perc.) in potatoes. J Genet 30:127–146
Bormann CA, Rickert AM, Ruiz RA, Paal J, Lübeck J, Strahwald J, Buhr K, Gebhardt C (2004) Tagging quantitative trait loci for maturity-corrected late blight resistance in tetraploid potato with PCR-based candidate gene markers. Mol Plant Microbe Interact 17:1126–1138
Botstein D, White RL, Skolnick M, Davis RW (1980) Construction of a genetic linkage map in man using restriction fragment length polymorphism. Am J Hum Genet 32:314–331
Brugmans B, Hutten R, Rookmaker A, Visser R, van Eck H (2006) Exploitation of a marker dense linkage map of potato for positional cloning of a wart disease resistance gene. Theor Appl Genet 112:269–277
Çakır E, van Leeuwen GCM, Flath K, Meffert JP, Janssen WAP, Maden S (2009) Identification of pathotypes of Synchytrium endobioticum found in infested fields in Turkey. Bull OEPP/EPPO Bull 39:175–178
Feingold S, Lloyd J, Norero N, Bonierbale M, Lorenzen J (2005) Mapping and characterization of new EST-derived microsatellites for potato (Solanum tuberosum L.). Theor Appl Genet 111:456–466
Gebhardt C (2007) Molecular markers, maps and population genetics. In: Vreudgenhil D, Bradshaw J, Gebhardt C, Govers F, MacKerron DKL, Taylor MA, Ross HA (eds) Potato biology and biotechnology, advances and perspectives. Elsevier, Amsterdam, pp 77–86
Gebhardt C, Bellin D, Henselewski H, Lehmann W, Schwarzfischer J, Valkonen JP (2006) Marker-assisted combination of major genes for pathogen resistance in potato. Theor Appl Genet 112:1458–1464
Gebhardt C, Valkonen JP (2001) Organization of genes controlling disease resistance in the potato genome. Annu Rev Phytopathol 39:79–102
Ghislain M, Núñez J, del Rosario Herrera M, Pignataro J, Guzman F, Bonierbale M, Spooner D (2009) Robust and highly informative microsatellite-based genetic identity kit for potato. Mol Breed 23:377–388
Glynne MD (1925) Infection experiments with wart disease of potatoes, Synchytrium endobioticum (Schilb.) Perc. Ann Appl Biol 12:34–60
Gupta P, Rustgi S, Kulwal P (2005) Linkage disequilibrium and association studies in higher plants: present status and future prospects. Plant Mol Biol 57:461–485
Hamada H, Petrino MG, Kakunaga T (1982) A novel repeated element with Z-DNA-forming potential is widely found in evolutionarily diverse eukaryotic genomes. Proc Natl Acad Sci USA 79:6465–6469
Hehl R, Faurie E, Hesselbach J, Salamini F, Whitham S, Baker B, Gebhardt C (1999) TMV resistance gene N homologues are linked to Synchytrium endobioticum resistance in potato. Theor Appl Genet 98:379–386
Laidlaw W (1985) A method for the detection of the resting sporangia of potato wart disease (Synchytrium endobioticum) in the soil of old outbreak sites. Potato Res 28:223–232
Langerfeld E, Stachewicz H (1994) Assessment of varietal reactions to potato wart (Synchytrium endobioticum) in Germany. EPPO Bull 24:793–798
Lemmerzahl J (1930) Neues vereinfachtes Infektionsverfahren zur Prüfung von Kartoffelsorten auf Krebsfestigkeit. Züchter 2:288–297
Lunden A, Jørstad I (1934) Investigations on the inheritance of immunity to wart disease (Synchytrium Endobioticum [Schilb.] Perc.) in the potato. J Genet 29:375–385
Maris B (1973) Studies with potato dihaploids on the inheritance of resistance to wart disease. Potato Res 16:324
Melnik PA (1998) Wart disease of potato, Synchytrium endobioticum (Schilbersky) Percival. In: EPPO technical documents, Paris, pp 880–884
Meyer S, Nagel A, Gebhardt C (2005) PoMaMo—a comprehensive database for potato genome data. Nucl Acids Res 33:D666–D670
Michelmore RW, Paran I, Kesseli RV (1991) Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc Natl Acad Sci USA 88:9828–9832
Milbourne D, Meyer RC, Collins AJ, Ramsay LD, Gebhardt C, Waugh R (1998) Isolation, characterisation and mapping of simple sequence repeat loci in potato. Mol Gen Genet 259:233–245
Mullis KB, Faloona FA (1987) Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 155:335–350
Pajerowska-Mukhtar K, Stich B, Achenbach U, Ballvora A, Lübeck J, Strahwald J, Tacke E, Hofferbert H-R, Ilarionova E, Bellin D, Walkemeier B, Basekow R, Kersten B, Gebhardt C (2009) Single nucleotide polymorphisms in the Allene Oxide Synthase 2 gene are associated with field resistance to late blight in populations of tetraploid potato cultivars. Genetics 181:1115–1127
Ross H (1986) Potato breeding—problems and perspectives. Paul Parey, Berlin, p 132
Salaman RN, Lesley JW (1923) Genetic studies in potatoes; the inheritance of immunity to wart resistance. J Genet 13:177–186
Sattarzadeh A, Achenbach U, Lubeck J, Strahwald J, Tacke E, Hofferbert HR, Rothsteyn T, Gebhardt C (2006) Single nucleotide polymorphism (SNP) genotyping as basis for developing a PCR-based marker highly diagnostic for potato varieties with high resistance to Globodera pallida pathotype Pa2/3. Mol Breed 18:301–312
Simko I, Jansky S, Stephenson S, Spooner D (2007) Genetics of resistance to pests and disease. In: Vreugdenhil D, Bradshaw J, Gebhardt C, Govers F, MacKerron DKL, Taylor MA, Ross HA (eds) Potato biology and biotechnology advances and perspectives. Elsevier, Amsterdam, pp 117–155
Stachewicz H (2002) Bedeutung und Bekämpfung des Kartoffelkrebses in Deutschland. Mitt. Biol. Bundesanst. Land-Forstwirtsch. 388:16–24
Stachewicz H, Enzian S (1998a) Bedeutung und Bekämpfung des Kartoffelkrebses (Synchytrium endobioticum) in der Bundesrepublik Deutschland. Mitt. Biol. Bundesanst. Land-Forstwirtsch. Berlin-Dahlem 357:102–103
Stachewicz H, Enzian S (1998b) Sind Temperatur und Niederschlagsmenge begrenzende Faktoren für das Auftreten von Kartoffelkrebs in der Bundesrepublik Deutschland? Nachrichtenbl. Deut. Pflanzenschutzd. 50:105–111
Stachewicz H, Flath K, Niepold F (2005) Methoden zur Bewertung der Kartoffelkrebsresistenz. Kartoffelbau 6:1–6
Szajko K, Chrzanowska M, Witek K, Strzelczyk-Żyta D, Zagórska H, Gebhardt C, Hennig J, Marczewski W (2008) The novel gene Ny-1 on potato chromosome IX confers hypersensitive resistance to Potato virus Y and is an alternative to Ry genes in potato breeding for PVY resistance. Theor Appl Genet 116:297–303
Tautz D, Renz M (1984) Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucl Acids Res 12:4127–4138
Tsumura Y, Ohba K, Strauss S (1996) Diversity and inheritance of inter-simple sequence repeat polymorphisms in Douglas-fir (Pseudotsuga menziesii) and sugi (Cryptomeria japonica). Theor Appl Genet 92:40–45
Vidal S, Cabrera H, Andersson RA, Fredriksson A, JPT- Valkonen (2002) Potato gene Y-1 is an N gene homolog that confers cell death upon infection with potato virus Y. Mol Plant Microbe Interact 15:717–727
Vos P, Hogers R, Bleeker M, Reijans M, van der Lee T, Fornes M, Frijters A, Pot J, Peleman J, Kuiper M, Zabeau M (1995) AFLP: a new technique for DNA fingerprinting. Nucl Acids Res 23:4407–4414
Whitham S, Dinesh-Kumar SP, Choi D, Hehl R, Corr C, Baker B (1994) The product of the tobacco mosaic virus resistance gene N: similarity to toll and the interleukin-1 receptor. Cell 78:1101–1115
Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucl Acids Res 18(22):6531–6535
Acknowledgments
This work was supported by the German Ministry for Research and Technology (BMBF) Program “Biochance Plus” grant 0313966 and the Max-Planck society. Part of this work was carried out in the department of Plant Breeding and Genetics headed by Maarten Koornneef. The technical assistance of Merle Noschinski, Sandra Mäurer and Verena Ristau is gratefully acknowledged. The authors thank H. Stachewicz and Mrs. Kerschnetzki for valuable help with the phenotyping of wart resistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Waugh.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Ballvora, A., Flath, K., Lübeck, J. et al. Multiple alleles for resistance and susceptibility modulate the defense response in the interaction of tetraploid potato (Solanum tuberosum) with Synchytrium endobioticum pathotypes 1, 2, 6 and 18. Theor Appl Genet 123, 1281–1292 (2011). https://doi.org/10.1007/s00122-011-1666-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-011-1666-9







