Abstract
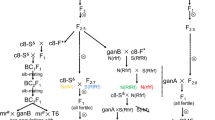
The present study was aimed at characterizing cytoplasmic male sterility (CMS) and identifying the fertility restorer gene for CMS (Diplotaxis catholica) Brassica juncea derived through sexual hybridization. The fertility restorer gene was identified by crossing the CMS line with progeny plants derived from somatic hybrids of B. juncea and D. cathoilca. The CMS line is comparable to the nuclear donor B. juncea in all respects except for flower and silique characteristics. In CMS plants, the flowers have smaller nectaries, and anthers are converted into petals or tubular structures. Gynoecium exhibits a crooked style and trilocular ovary. Seed fertility was reduced in the CMS line. Genetic segregation data indicated that a single, dominant, nuclear gene governs fertility restoration. Restored plants showed a high female fertility and lacked gynoecium abnormalities. In fertility-restored plants, petal development was found to be variable; some flowers had the normal number of four petals, while others had zero to three petals. Interestingly, the trilocular character of the ovary was found to co-segregate with CMS and became bilocular upon male-fertility restoration. Thus, this trait appears to be affected by the interaction of nuclear and mitochondrial (mt) genomes. Restriction fragment length polymorphism analysis indicated that mt-genome of D. catholica is highly divergent from that of B. juncea. However, in Northern analysis, out of eight mt genes studied, an altered transcript pattern was recorded for only atpA. In fertility-restored plants, the atpA transcript became shorter, thereby showing its association with CMS.


Similar content being viewed by others
References
Abad AR, Mehrtens BJ, Mackenzie SA (1995) Specific expression in reproductive tissues and fate of a mitochondrial sterility associated protein in cytoplasmic male-sterile bean. Plant Cell 7:271–285
Alt J, Morris J, Westhoff P, Herrmann RG (1984) Nucleotide sequence of the clustered genes for 44KD chlorophyll apoprotein and the 32KD protein of the photosystem II reaction center in the spinach plastid chromosome. Curr Genet 8:597–606
Arumugam N, Mukhopadhyay A, Gupta V, Pental D, Pradhan AK (1996) Synthesis of hexaploid (aabbcc) somatic hybrids: a bridging material for transfer of Tour cytoplasmic male sterility to different Brassica species. Theor Appl Genet 92:762–768
Bellaoui M, Grelon M, Pelletier G, Budar F (1999) The restorer Rfo gene acts post-transcriptionally on the stability of the ORF138 Ogura CMS-associated protein in reproductive tissues of rapeseed cybrids. Plant Mol Biol 40:893–902
Bentolila S, Alfonso AA, Hanson MR (2002) A pentatricopeptide repeat-containing gene restores fertility to cytoplasmic male-sterile plants. Proc Natl Acad Sci USA 99:10,887–10,892
Bergman P, Edqvist J, Farbos I, Glimelius K (2000) Male-sterile tobacco displays abnormal mitochondrial atp1 transcript accumulation and reduced floral ATP/ADP ratio. Plant Mol Biol 42:531–544
Bhat SR, Haque A, Chopra VL (2001) Induction of mutations for cytoplasmic male sterility and some rare phenotypes in Indian mustard (Brassica juncea L.). Indian J Genet 61:335–340
Braun CJ, Levings CS III (1985) Nucleotide sequence of the Fo-ATPase – a subunit gene from maize mitochondria. Plant Physiol 79:571–577
Brown GG (1999) Unique aspects of cytoplasmic male sterility and fertility restoration in Brassica napus. J Hered 90:351–356
Dawson AJ, Jones VP, Leaver CJ (1984) The apocytochrome b gene in maize mitochondria does not contain introns and is preceded by a potential ribosome binding site. EMBO J 3:2107–2113
Delourme R, Eber F, Renard M (1991) Radish cytoplasmic male sterility in rapeseed: Breeding restorer lines with a good female fertility. In: McGregor DI (ed) 8th Int Rapeseed Congr. University Extension Press, Saskatoon, Sask, pp 1506–1510
Dewey RE, Levings CS III, Timothy DH (1985a) Nucleotide sequence of ATPase subunit 6 gene of maize mitochondria. Plant Physiol 79:914–919
Dewey RE, Schuster AM, Levings CS III, Timothy DH (1985b) Nucleotide sequence of Fo–ATPase proteolipid (subunit 9) gene of maize mitochondria. Proc Natl Acad Sci USA 84:5374–5378
Dill CL, Wise RP, Schnable PS (1997) Rf8 and Rf* mediate unique T-urf-13 transcript accumulation, revealing a conserved motif associated with RNA processing and restoration of fertility in T-cytoplasm maize. Genetics 147:1367–1379
Fox TD, Leaver CJ (1981) The Zea mays mitochondrial gene coding cytochrome oxidase subunit II has an intervening sequence and does not contain TGA codon. Cell 26:315–323
Fu TD (1981) Production and research on rapeseed in the Peoples Republic in China. Cruciferae Newslett 6:6–7
Grelon M, Budar F, Bonhomme S, Pelletier G (1994) Ogura cytoplasmic male-sterile (CMS)-associated orf138 is translated into a mitochondrial membrane polypeptide in male-sterile Brassica cybrids. Mol Gen Genet 243:540–547
Gwynn B, Dewey RE, Scderoff RR, Timothy DM, Levings CS III (1987) Sequence of the 18S ribosomal gene region and the cytochrome oxidase II gene from the mt DNA of Zea diploperennis. Theor Appl Genet 41:781–788
Handa H, Nakajima K (1991) Nucleotide sequence and transcription analysis of the rapeseed (Brassica napus L.) mitochondrial F1-ATPase α-subunit gene. Plant Mol Biol 16:361–364
Hanson MR (1991) Plant mitochondrial mutations and male sterility. Annu Rev Genet 25:461–486
Issac PG, Jones VP, Leaver CJ (1985) The maize cytochrome oxidase subunit gene sequence, expression and rearrangement in cytoplasmic male sterile plants. EMBO J 12:1617–1623
Iwabuchi M, Kyozuka J, Shimamoto K (1993) Processing followed by complete editing of an altered mitochondrial atp6 RNA restores fertility of cytoplasmic male sterile rice. EMBO J 12:1437–1446
Kempken F, Pring DR (1999) Male sterility in higher plants – fundamentals and applications. Prog Bot 60:140–166
Kennel JC, Pring DR (1989) Initiation and processing of atp6, T-urf13 and orf221 transcripts from T cytoplasm maize. Mol Gen Genet 216:16–24
Kirti PB, Mohapatra T, Baldev A, Prakash S, Chopra VL (1995a) A stable cytoplasmic male sterile line of Brassica juncea carrying restructured organelle genomes from the somatic hybrid of Trachystoma balli + B. juncea. Plant Breed 114:434–438
Kirti PB, Mohapatra T, Khanna H, Prakash S, Chopra VL (1995b) Diplotaxis catholica + Brassica juncea somatic hybrids: molecular and cytogenetic characterization. Plant Cell Rep 14:593–597
Kirti PB, Baldev A, Gaikwad K, Bhat SR, Dinesh Kumar V, Prakash S, Chopra VL (1997) Introgression of a gene restoring fertility to CMS (Trachystoma) Brassica juncea and the genetics of restoration. Plant Breed 116:259–262
Krishnasamy S, Makaroff CA (1993) Characterization of the radish mitochondrial orfB locus: possible relationship with male-sterility in Ogura radish. Curr Genet 24:156–163
Landgren M, Zetterstrand M, Sundberg E, Glimelius K (1996) Alloplasmic male sterile Brassica lines containing B. tournefortii mitochondria express an orf 3′ of the atp6 gene and a 32KD protein. Plant Mol Biol 32:870–890
L'Homme Y, Stahl R, Li X-Q, Hameed A, Brown GG (1997) Brassica 'nap' cytoplasmic male sterility is associated with expression of a mtDNA region containing a chimeric gene similar to the 'pol' CMS-associated orf224 gene. Curr Genet 31:325–335
Liu J-H, Landgren M, Glimelius K (1996) Transfer of Brassica tournefortii cytoplasm to Brassica napus for the production of cytoplasmic male sterile B. napus. Physiol Plant 96:123–129
Makaroff CA, Palmer JD (1988) Mitochondrial DNA rearrangements and transcriptional alterations in the male-sterile cytoplasm of Ogura radish. Mol Cell Biol 8:1474–1480
Malik M, Vyas P, Rangaswamy NS, Shivanna KR (1999) Development of two new cytoplasmic male-sterile lines of Brassica juncea through wide hybridization. Plant Breed 118:75–78
Mohanty A (1996) Hybridisation between crop Brassicas and some of their wild allies. PhD thesis, University of Delhi, India
Mohapatra T, Kirti PB, Kumar VD, Prakash S, Chopra VL (1998) Random chloroplast segregation and mitochondrial genome recombination in somatic hybrid plants of Diplotaxis catholica and Brassica juncea. Plant Cell Rep 17:814–818
Nei M, Li WH (1979) Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc Natl Acad Sci USA 76:5269–5273
Ogihara Y, Kurihara Y, Futami K, Tsuji K, Murai K (1999) Photoperiod-sensitive cytoplasmic male sterility in wheat nuclear mitochondrial incompatibility results in differential processing of the mitochondrial orf25 gene. Curr Genet 36:354–362
Ogura H (1968) Studies on the new male-sterility in Japanese radish with specific reference to the utilization of this sterility towards the practical raising of hybrid seeds. Mem Fac Agric Kagoshima Univ 6:39–78
Pellan-Delourme R, Renard M (1988) Cytoplasmic male sterility in rapeseed (Brassica napus L.). Female fertility of restored rapeseed with ogura and hybrid cytoplasms. Genome 30:234–238
Pradhan AK, Prakash S, Mukhopadhyay A, Pental D (1992) Phylogeny of Brassica and allied genera based on variations in chloroplast and mitochondrial DNA patterns: molecular and taxonomic classifications are incongruous. Theor Appl Genet 85:331–340
Prakash S, Chopra VL (1990) Male sterility caused by cytoplasm of Brassica oxyrrhina in B. campestris and B. juncea. Theor Appl Genet 79:285–287
Prakash S, Kirti PB, Bhat SR, Gaikwad K, Kumar VD, Chopra VL (1998) A Moricandia arvensis-based cytoplasmic male sterility and fertility restoration system in Brassica juncea. Theor Appl Genet 97:488–492
Prakash S, Ahuja I, Upreti HC, Dinesh Kumar V, Bhat SR, Kirti PB, Chopra VL (2001) Expression of male sterility in allplasmic Brassica juncea with Erucastrum canariense cytoplasm and the development of a fertility restorer system. Plant Breed 120:479–482
Pruitt KD, Hanson MR (1991) Transcripts of the Petunia mitochondrial CMS-associated pcf locus in male sterile and fertility-restored lines. Mol Gen Genet 227:348–355
Rao GU, Batra-Sarup V, Prakash S, Shivanna KR (1994) Development of a new cytoplasmic male sterility system in Brassica juncea through wide hybridization. Plant Breed 12:909–912
Sakamoto W, Kondo H, Murata M, Motoyoshi F (1996) Altered mitochondrial gene expression in a maternally distorted leaf mutant of Arabidopsis induced by chloroplast mutator. Plant Cell 8:1377–1390
Schnable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180
Shiga T, Baba S (1971) Cytoplasmic male sterility in rape plants (Brassica napus L.). Jpn J Breed 21:16–17
Singh M, Brown GG (1991) Suppression of cytoplasmic male sterility by nuclear genes alters expression of a novel mitochondrial gene region. Plant Cell 3:1349–1362
Sodhi YS, Pradhan AK, Verma JK, Arumugam N, Mukhopadhyay A, Pental D (1994) Identification and inheritance of fertility restorer genes for 'tour' CMS in rapeseed (Brassica napus L.). Plant Breed 112:223–227
Ullstrup AJ (1972) The impacts of the Southern corn leaf blight epidemics of 1970–71. Annu Rev Phytopathol 10:37–50
Wen L, Chase CD (1999) Pleiotropic effects of a nuclear restorer-of-fertility locus on mitochondrial transcripts in male-fertile and S male-sterile maize. Curr Genet 35:521–526
Zurawaski G, Bohnert HJ, Whitefield PR, Bottomley Y (1982) Nucleotide sequence of the gene for the Mr 32000 thylakoid membrane protein from Spinacea oleracea and Nicotiana debneyi product of Mr 38950. Proc Natl Acad Sci USA 79:7699–7703
Acknowledgements
We are grateful to Prof. K. Shivanna, University of Delhi, India for providing the seeds of the CMS line. AP was supported by a student grant from the Council of Scientific and Industrial Research, Government of India.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Glimelius
Rights and permissions
About this article
Cite this article
Pathania, A., Bhat, S.R., Dinesh Kumar, V. et al. Cytoplasmic male sterility in alloplasmic Brassica juncea carrying Diplotaxis catholica cytoplasm: molecular characterization and genetics of fertility restoration. Theor Appl Genet 107, 455–461 (2003). https://doi.org/10.1007/s00122-003-1266-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-003-1266-4