Abstract
Background
High-risk drug consumption is a matter of great concern for public health actors in industrialised countries. The latest trends show a market tendency towards diversification and increasing demand for high-purity synthetic drugs. While most consumers seek medical help after cannabis use, it is high-risk drugs like cocaine, heroin and amphetamines that account for most of the 1000 drug-related deaths that occur in Germany every year.
Purpose
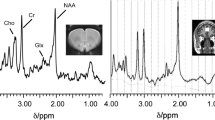
This article presents the most prominent in vivo cerebral metabolic information in cocaine, heroin and methamphetamine users provided by MRI spectroscopy and PET imaging.
Materials and methods
We reviewed the literature reporting neuroimaging studies of in vivo metabolic data for methamphetamine, cocaine and heroin consumption published up to March 2017. The search was conducted using PubMed and a combination of the following key words: methamphetamine, cocaine, heroin, MR spectroscopy, PET.
Conclusion
MRI and PET are indispensable tools in gauging brain metabolic response to illegal drug abuse. Future breakthroughs in this field will most likely come from the investigation of novel neurotransmitter systems in PET and imaging phosphorus and carbon metabolites in MRI.
Zusammenfassung
Hintergrund
In den Industrieländern ist der Konsum von Hochrisikodrogen ein erhebliches Problem für das gesamte Gesundheitssystem. Neueste Entwicklungen zeigen eine Tendenz zu immer größerer Diversifikation und eine erhöhte Nachfrage nach synthetischen Drogen von hohem Reinheitsgrad. Während die meisten Konsumenten medizinische Hilfe nach Cannabisverbrauch suchen, sind es die Hochrisikodrogen wie Heroin, Kokain und Amphetamine, an denen die meisten der 1000 Drogentoten pro Jahr in Deutschland sterben.
Ziel
In diesem Artikel werden die auffälligsten, mittels Magnetresonanzspektroskopie (MRS) und Positronenemissionstomographie (PET) erfassten In-vivo-Daten zum Hirnstoffwechsel bei Konsumenten von Kokain, Heroin und Methamphetaminen vorgestellt.
Material und Methoden
Die Literatur über den zerebralen Energiestoffwechsel bei Methamphetamin‑, Kokain- und Heroinkonsumenten bis März 2017 wurde über PubMed-Recherchen erforscht. Suchbegriffe wie „methamphetamine“, „cocaine“, „heroin“, „MR spectroscopy“, „PET“ wurden benutzt.
Schlussfolgerung
MRT- und PET-Verfahren sind unerlässliche Werkzeuge bei der Erforschung der zerebralen Reaktionen auf illegale Drogen. Zukünftige Durchbrüche werden voraussichtlich durch die Untersuchung neuer Neurotransmittersysteme in der PET sowie die Abbildung von Phosphor- und Kohlenstoffmetaboliten in der MRT erfolgen.



Similar content being viewed by others
Literatur
Büttner A (2011) Review: the neuropathology of drug abuse. Neuropathol Appl Neurobiol 37:118–134. doi:10.1111/j.1365-2990.2010.01131.x
Chang L, Ernst T, Strickland T, Mehringer CM (1999) Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry 156:716–722. doi:10.1176/ajp.156.5.716
Christensen JD, Kaufman MJ, Levin JM et al (1996) Abnormal cerebral metabolism in polydrug abusers during early withdrawal: a 31P MR spectroscopy study. Magn Reson Med 35:658–663
Christensen JD, Kaufman MJ, Frederick B et al (2000) Proton magnetic resonance spectroscopy of human basal ganglia: response to cocaine administration. Biol Psychiatry 48:685–692
ESPAD (2015) ESPAD Report 2015 doi:10.2810/86718
European Monitoring Centre for Drugs and Drug Addiction (2014) European Drug Report 2014: Trends and developments. Report doi:10.2810/32306
Fowler JS, Volkow ND, Wang GJ et al (2001) [11]Cocaine: PET studies of cocaine pharmacokinetics, dopamine transporter availability and dopamine transporter occupancy. Nucl Med Biol 28:561–572. doi:10.1016/S0969-8051(01)00211-6
Gururajan A, Manning EE, Klug M, van den Buuse M (2012) Drugs of abuse and increased risk of psychosis development. Aust N Z J Psychiatry 46:1120–1135. doi:10.1177/0004867412455232
Halpin LE, Collins SA, Yamamoto BK (2014) Neurotoxicity of methamphetamine and 3,4-methylenedioxymethamphetamine. Life Sci 97:37–44. doi:10.1016/j.lfs.2013.07.014
Hermann D, Frischknecht U, Heinrich M et al (2012) MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol 17:659–667. doi:10.1111/j.1369-1600.2010.00290.x
Hou H, Wang C, Jia S et al (2014) Brain dopaminergic system changes in drug addiction: a review of positron emission tomography findings. Neurosci Bull 30:765–776. doi:10.1007/s12264-014-1469-5
Howells FM, Uhlmann A, Temmingh H et al (2014) 1H-magnetic resonance spectroscopy (1H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res 153:122–128. doi:10.1016/j.schres.2014.01.029
Hulka LM, Scheidegger M, Vonmoos M et al (2016) Glutamatergic and neurometabolic alterations in chronic cocaine users measured with 1H-magnetic resonance spectroscopy. Addict Biol 21:205–217. doi:10.1111/adb.12217
Jan RK, Kydd RR, Russell BR (2012) Functional and structural changes associated with methamphetamine abuse. Brain Sci 2:434–482. doi:10.3390/brainsci2040434
Kaufman MJ, Pollack MH, Villafuerte RA et al (1999) Cerebral phosphorus metabolite abnormalities in opiate-dependent polydrug abusers in methadone maintenance. Psychiatry Res 90:143–152
Ke Y, Streeter CC, Nassar LE et al (2004) Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J‑resolved magnetic resonance spectroscopy study. Psychiatry Res 130:283–293. doi:10.1016/j.pscychresns.2003.12.001
Kim JE, Kim GH, Hwang J et al (2016) Metabolic alterations in the anterior cingulate cortex and related cognitive deficits in late adolescent methamphetamine users. Addict Biol. doi:10.1111/adb.12473
Li SJ, Wang Y, Pankiewicz J, Stein EA (1999) Neurochemical adaptation to cocaine abuse: Reduction of N‑acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry 45:1481–1487. doi:10.1016/S0006-3223(98)00230-3
Licata SC, Renshaw PF (2010) Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci 1187:148–171. doi:10.1111/j.1749-6632.2009.05143.x
London ED, Stapleton JM, Phillips RL et al (1996) PET studies of cerebral glucose metabolism: acute effects of cocaine and long-term deficits in brains of drug abusers. NIDA Res Monogr 163:146–158
Martinez D, Saccone PA, Liu F et al (2012) Deficits in dopamine D 2 receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry 71:192–198. doi:10.1016/j.biopsych.2011.08.024
Martinez D, Slifstein M, Nabulsi N et al (2014) Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry 75:165–171. doi:10.1016/j.biopsych.2013.06.026
Matuskey D, Gallezot JD, Pittman B et al (2014) Dopamine D3 receptor alterations in cocaine-dependent humans imaged with [11C](+)PHNO. Drug Alcohol Depend 139:100–103. doi:10.1016/j.drugalcdep.2014.03.013
Moreno-López L, Stamatakis EA, Fernández-Serrano MJ et al (2012) Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: a resting-PET brain metabolism study. PLOS ONE. doi:10.1371/journal.pone.0039830
Mostardt S, Floter S, Neumann A, Wasem J (2010) Public expenditure caused by the consumption of illicit drugs in Germany. Gesundheitswesen 72:886–894. doi:10.1055/s-0029-1243212
Nordahl TE, Salo R, Natsuaki Y et al (2005) Methamphetamine users in sustained abstinence. Arch Gen Psychiatry 62:444–452. doi:10.1001/archpsyc.62.4.444
Offiah C, Hall E (2008) Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol 63:146–152. doi:10.1016/j.crad.2007.07.021
Parrott AC (2015) Why all stimulant drugs are damaging to recreational users: an empirical overview and psychobiological explanation. Hum Psychopharmacol 30:213–224. doi:10.1002/hup.2468
Shi J, Zhao LY, Copersino ML et al (2008) PET imaging of dopamine transporter and drug craving during methadone maintenance treatment and after prolonged abstinence in heroin users. Eur J Pharmacol 579:160–166. doi:10.1016/j.ejphar.2007.09.042
Smith LM, Chang L, Yonekura ML et al (2001) Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics 107:227–231
Streeter CC, Hennen J, Ke Y et al (2005) Prefrontal GABA levels in cocaine-dependent subjects increase with pramipexole and venlafaxine treatment. Psychopharmacology (Berl) 182:516–526. doi:10.1007/s00213-005-0121-5
Sung YH, Carey PD, Stein DJ et al (2013) Decreased frontal N‑acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behav Brain Res 246:154–161. doi:10.1016/j.bbr.2013.02.028
Sung YH, Yurgelun-Todd DA, Shi XF et al (2013) Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Depend 129:102–109. doi:10.1016/j.drugalcdep.2012.09.015
Volkow ND, Fowler JS, Wolf AP et al (1991) Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry 148:621–626. doi:10.1176/ajp.148.5.621
Volkow ND, Hitzemann R, Wang G‑J et al (1992) Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11:184–190. doi:10.1002/syn.890110303
White JM, Irvine RJ (1999) Mechanisms of fatal opioid overdose. Addiction 94:961–972. doi:10.1046/j.1360-0443.1999.9479612.x
Worhunsky PD, Matuskey D, Gallezot J‑D et al (2017) Regional and source-based patterns of [11C]-(+)-PHNO binding potential reveal concurrent alterations in dopamine D2 and D3 receptor availability in cocaine-use disorder. Neuroimage 148:343–351. doi:10.1016/j.neuroimage.2017.01.045
Yang S, Salmeron BJ, Ross TJ et al (2009) Lower glutamate levels in rostral anterior cingulate of chronic cocaine users – A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res 174:171–176. doi:10.1016/j.pscychresns.2009.05.004
Yu S, Zhu L, Shen Q et al (2015) Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol. doi:10.1155/2015/103969
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. Bodea declares that he has no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
The supplement containing this article is not sponsored by industry.
Rights and permissions
About this article
Cite this article
Bodea, S. CNS metabolism in high-risk drug abuse. Radiologe 58 (Suppl 1), 34–39 (2018). https://doi.org/10.1007/s00117-017-0255-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00117-017-0255-6