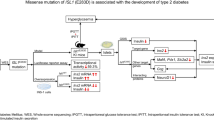
Abstract
Progranulin is a glycoprotein marking chronic inflammation in obesity and type 2 diabetes. Previous studies suggested PSRC1 (proline and serine rich coiled-coil 1) to be a target of genetic variants associated with serum progranulin levels. We aimed to identify potentially functional variants and characterize their role in regulation of PSRC1. Phylogenetic module complexity analysis (PMCA) prioritized four polymorphisms (rs12740374, rs629301, rs660240, rs7528419) altering transcription factor binding sites with an overall score for potential regulatory function of Sall > 7.0. The effects of these variants on transcriptional activity and binding of transcription factors were tested by luciferase reporter and electrophoretic mobility shift assays (EMSA). In parallel, blood DNA promoter methylation of two regions was tested in subjects with a very high (N = 100) or a very low (N = 100) serum progranulin. Luciferase assays revealed lower activities in vectors carrying the rs629301-A compared with the C allele. Moreover, EMSA indicated a different binding pattern for the two rs629301 alleles, with an additional prominent band for the A allele, which was finally confirmed with the supershift for the Yin Yang 1 transcription factor (YY1). Subjects with high progranulin levels manifested a significantly higher mean DNA methylation (P < 1 × 10−7) in one promoter region, which was in line with a significantly lower PSRC1 mRNA expression levels in blood (P = 1 × 10−3). Consistently, rs629301-A allele was associated with lower PSRC1 mRNA expression (P < 1 × 10−7). Our data suggest that the progranulin-associated variant rs629301 modifies the transcription of PSRC1 through alteration of YY1 binding capacity. DNA methylation studies further support the role of PSRC1 in regulation of progranulin serum levels.
Key messages
-
PSRC1 (proline and serine rich coiled-coil 1) SNPs are associated with serum progranulin levels.
-
rs629301 regulates PSRC1 expression by affecting Yin Yang 1 transcription factor (YY1) binding.
-
PSRC1 is also epigenetically regulated in subjects with high progranulin levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Progranulin (PGRN) is a glycoprotein with a wide range of functions involved, e.g., in inflammatory pathways, metabolism, cell proliferation, and lysosome regulation [1,2,3]. PGRN is encoded by the GRN gene [4], whose mutations can cause frontotemporal lobar degeneration and neuronal ceroid lipofuscinosis [5, 6], but may potentially be also involved in the pathogenesis of Alzheimer’s disease [6,7,8,9].
Recent genome-wide association studies identified several loci associated with serum progranulin levels. The most prominent are the CELSR2-PSRC1-MYBPHL-SORT1, the CDH23-PSAP, and the GRN locus [10,11,12]. In particular, the Sortilin 1 gene (SORT1) has been extensively studied as a target gene of the associated variants and shown to regulate circulating low-density lipoprotein levels influencing risk of cardiovascular diseases [13, 14]. SORT1 is affected by several microRNAs, e.g., miR-146a and miR-182, and is considered to play a role in arterial calcification and chronic inflammation in endothelial cells [15,16,17].
Besides SORT1, PSRC1 (proline and serine rich coiled-coil 1) has been suggested by recent data including eQTLs (expression quantitative trait locus) [11] to be a target gene of single-nucleotide polymorphisms (SNPs) associated with serum progranulin levels. Furthermore, gene silencing experiments demonstrated the role of PSRC1 in regulation of progranulin secretion in vitro; however, the functional variant and underlying molecular mechanism have not yet been clarified.
In the present study, we therefore performed in silico and in vitro experiments to identify the potentially causal variants for increased serum progranulin levels and to elucidate their role in transcriptional and epigenetic regulation of PSRC1.
Material and methods
The Sorbs cohort
A total of 200 individuals with a mean BMI of 26.0 ± 4.2 kg/m2 and a mean age of 46 ± 16 years were included in the PSRC1 promoter methylation analysis. They are part of a metabolically well-characterized cohort of Sorbs from Eastern Germany that was extensively phenotyped for a wide range of anthropometric and metabolic traits (Supplementary Table 1) [18, 19]. The 200 subjects were selected according to their maximal distance in progranulin serum levels, building one group with very high (mean ± SD 151.98 ± 20.86 ng/ml; N = 100) and the other one with very low (mean ± SD 73.98 ± 10.04 ng/ml; N = 100) concentrations. In addition, both groups were matched for age and BMI by filtering the groups using t-statistics, and for gender and smoking status by conducting a chi-square test. Subjects with diabetes were not included in the present analysis. All participants gave their written informed consent, and the study was approved by the ethics committee of the University of Leipzig.
Detailed description of all study participants is provided in the Supplemental Table 1.
Functional annotation
Functional annotation for all SNPs in linkage disequilibrium (LD, defined as r2 ≥ 0.86 in Europeans of the 1000 Genomes Phase 1 data) with the leading SNP (rs660240) from the initial GWAS (genome-wide association study) for progranulin serum levels [11] was performed using the phylogenetic module complexity analysis (PMCA) [20] by Genomatix GmbH (München, Germany). This method was used to narrow down potentially causal cis-regulatory variants for further functional analysis in vitro.
Cell culture
HepG2 and HeLa cell lines (ATCC; Manassas, Virginia) were used for all in vitro studies. Cells were maintained in Dulbeccos Modified Eagle Medium (DMEM; Gibco; 5.56-mM glucose, 1-mM pyruvate, 4-mM L-glutamine, ThermoFisher Scientific, Germany) supplemented with 10% fetal bovine serum (Biochrom GmbH, Germany).
Preparation of reporter constructs
Single-stranded oligonucleotides harboring each SNP as well as a Xho I restriction site on the 5′ and 3′ ends were purchased from MWG-Biotech (Ebersberg, Germany). Oligo-sequences are given in Table 1. Complementary oligos were annealed. Annealed oligos were digested with Xho I and cloned to the minimal promotor containing firefly luciferase vector pGL4.23.
Luciferase reporter assays
Functional relevance of candidate SNPs (rs12740374, rs629301, rs660240, rs7528419; all LDs r2 > 0.94) on transcriptional activity was evaluated by luciferase assay. HepG2 as well as HeLa cells were transfected for the luciferase assay. Cells were co-transfected with luciferase reporter constructs and the Renilla luciferase vector pGL4.74 as internal control using Fugene HD (Promega, Madison, WI) according to the manufacturer’s procedures. After 48 h, cells were harvested and luciferase activity was measured using the Dual Luciferase System (Promega, Madison, WI) as described in the manufacturer’s instructions. Ratio of firefly to Renilla luciferase was calculated. Assays were performed at least in triplicates, and values were normalized to pGL4.23/pGL4.74 empty control.
Electrophoretic mobility shift assay
The JASPAR (http://jaspar.genereg.net) and PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3) online databases were used to predict transcription factor binding to alleles of candidate SNPs. Nuclear protein was extracted from HeLa cells. IRDye 700 (SNPs) or IRDye 800 (positive control) labeled single-stranded oligos were purchased from metabion (Planegg, Germany). Oligo-sequences are given in Table 1. Oligos were annealed to generate double-stranded probes. Electrophoretic mobility shift assay (EMSA) was conducted as follows. Each reaction contained of 7-μg nuclear extract, 4-nmol probe, 1 X binding buffer (10-mM TRIS, 50-mM NaCl, pH 7.5), 2-mM dithiothreitol, 1-μg hering sperm DNA, and 0.25% Tween 20. Binding reaction was performed 30 min at room temperature. For supershift reactions, 4 μg of anti-Yin Yang 1 (YY1) (clone H-10, Santa Cruz Biotechnology Inc., Dallas, TX) was added to the reaction mixture and reactions were incubated for further 30 min. Samples were separated on a 4% native polyacrylamide gel in 0.5 X TRIS-Borate-EDTA (45-mM TRIS, 45-mM boric acid, 1-mM EDTA) followed by visualization with an Odyssey Infrared Imager (LI-COR Biosciences, Lincoln, NE).
DNA methylation analysis
In parallel, DNA methylation analyses were performed for two sequence segments within the PSRC1 promoter region (PyroMark assay 1 5′-3′UTR: TCTCCGCGCACGCGAGCACGCGCACTCGCAGCCTCAACCCTCGGCTCCGCCACCGGGATGCAGTCTTCTG, PyroMark assay2 5′-3′UTR: ACCGTTCTGGAGACTGGGTGCTCGGCGGCCCAGCAGAGGG AGCGGGG) using the pyrosequencing technique as described elsewhere [21] (Qiagen, Hilden, Germany) to test potential epigenetic differences in the PSRC1 promoter locus between subject with high vs. low progranulin serum levels. PyroMark assays were designed using the PyroMark Assay Design software 2.0 (Qiagen, Hilden, Germany). Briefly, target region was virtually bisulfite converted and primers were selected according to program-specific quality criteria. All reactions were performed in duplicates including two non-template controls per plate and sequenced on PyroMark Q24 (Qiagen, Hilden, Germany).
rs629301 genotype data
Based on i) our previous GWAS [11] which revealed rs629301 (A>C; MAF = 0.24) to be associated with serum progranulin and as a strong eQTL for the PSRC1 gene locus, and ii) present findings from the functional annotation studies using PMCA (see below in the “Results” section) further supporting the functional role of this variant, we focused on rs629301 for downstream functional analysis. Genotype data was available from a genome-wide data set for 977 individuals from the German population of Sorbs [22]. Rs629301 genotype distribution (AA/AC/CC: 616/322/39) was in Hardy-Weinberg equilibrium (P = 0.7).
Statistical analysis
CpG methylation levels per site were used as continuous variables, and mean levels were calculated per assay. All analyses were performed using R [23]. Wilcoxon rank sum t test was used to compare differences between subjects showing high vs. low progranulin serum levels. A chi-square test was used to estimate differences in the genotype distribution between both groups. Linear regression was applied to detect differences of PSRC1 mRNA expression levels (available from previous studies) [11] between the different genotypes using additive mode of inheritance (AA vs. AC vs. CC coded as 0 vs. 1 vs. 2, respectively) including adjustments for age and sex.
Statistical analyses for in vitro experiments were performed using the Graphpad Prism software version 6 (Graphpad Software Inc., San Diego, CA). Differences in luciferase assays were assessed by one-way ANOVA followed by Dunnett’s post hoc test to account for multiple comparisons. A P value of < 0.05 was considered as statistically significant in all analyses.
Results
Phylogenetic module complexity analysis identifies four genetic variants with potential regulatory function
Ten SNPs in tight LD (defined as r2 ≥ 0.86 in Europeans; Supplementary Table 3) with the GWAS lead SNP rs629301, previously shown to be associated with progranulin serum level [11], were functionally annotated using the PMCA [20] method to identify cis-regulatory variants, most likely affecting PSRC1 gene expression (Table 2). Among the 10 analyzed variants, we identified 4 SNPs (rs12740374, rs629301, rs660240, and rs7528419) with an overall score for the prediction of a potential regulatory region of Sall > 7.0, suggesting that these variants are classified and belong to a complex region [20]. The highest PMCA score was found for rs12740374 (Sall = 9.0, Table 1).
rs629301 alters gene expression in vitro
Functional relevance of the 4 candidate SNPs (rs12740374, rs629301, rs660240, rs7528419) on transcriptional activity was tested by using luciferase reporter assays. We observed significant differences in luciferase activities in both HepG2 and HeLa cells only for rs629301. Luciferase activities were lower in pGL4-rs629301A compared with pGL4-rs629301C allele carrying vector (Fig. 1 a and b). In addition, for rs12740374, the pGL4-rs12740374T showed higher luciferase activity than the pGL4-rs12740374G vector, although both alleles appeared to increase luciferase activities when compared with control vectors (Fig. 1 a and b).
Transcription activity of four candidate SNPs using luciferase assay. Promotor activity of firefly luciferase levels is shown relative to Renilla luciferase. Values are normalized to the luciferase levels of pGL4.23/pGL4.74 empty vector. Three replicate experiments were performed in duplicates, mean ± SD is shown. Analysis was done by one-way ANOVA followed by Dunnett’s post hoc Test.*P < 0.05, **P < 0.01, ***P < 0.001, ***P < 0.0001. ns, not significant. a Luciferase activity in HepG2 cells. b Luciferase activity in HeLa cells
rs629301 affects binding of Yin Yang 1 regulating the transcription of PSRC1
Based on findings from luciferase assays, we next tested the binding of the rs629301 and rs12740374 minor and major alleles and demonstrated DNA-protein complexes only using oligo (A) for the major allele sequence of the rs629301 (Fig. 2). In summary, similar protein-binding pattern for the two rs12740374 alleles was observed (Fig. 2). In contrast, a different binding pattern for the two rs629301 alleles was found, with an additional prominent band for the A allele (Fig. 2). Moreover, JASPAR and PROMO databases suggested YY1 transcription factor consensus binding site around rs629301 (Fig. 3a). Addition of YY1 antibody led to a supershift of the prominent band observed in the rs629301 A allele (Fig. 3b). No supershifting for binding in the major and minor allele sequences of rs12740374 (data not shown) and minor allele of the rs629301 was present (Fig. 3b).
PSRC1 DNA methylation is increased in subjects with high serum progranulin levels
The subpopulation of individuals selected according to their either very high (N = 100, mean ± SD 151.98 ± 20.86 ng/ml) or very low (N = 100, mean ± SD 73.98 ± 10.04 ng/ml) serum progranulin level (Fig. 4a, P < 1 × 10−15) revealed a significant inverse difference in the PSRC1 mRNA expression levels (Fig. 4b, P = 1 × 10−3). PSRC1 promoter methylation for assay 1 (Fig. 4c) is in line with this and shows a significantly (P < 1 × 10−7) higher mean methylation level in “high progranulin” subjects. The second analyzed assay within the PSRC1 promoter did not show significant differences between the progranulin groups (data not shown).
Progranulin levels and epigenetic regulation of PSRC1 in the Sorbs population (N = 200). a The progranulin serum level in ng/ml. b The relative mRNA expression values of PSRC1. c The corresponding DNA methylation levels for PSRC1 assay 1 represented in %. All data in a–c is shown as scatter dot plots representing mean ± SD. **P < 0.01, ***P < 0.001
Rs629301 is associated with PSRC1 mRNA expression and serum progranulin levels
Distribution of rs629301 genotypes between the progranulin groups (“high” AA = 76, AC = 18, CC = 0; “low” AA = 36; AC = 44; CC = 14) clearly indicated overrepresentation of the A allele in the “high” progranulin group (Fig. 5a, P < 1 × 10−8). Again, inverse with the protein levels, the A allele was significantly associated with lower PSRC1 mRNA expression (Fig. 5b, P < 1 × 10−7, additive mode of inheritance), whereas, albeit not significant (Fig. 5c), PSRC1 methylation levels were increased.
Genotype effects on progranulin and PSRC1 regulation in the Sorbs population (N = 200). a The genotype distribution between subjects showing a high (AA = 76, AC = 18, CC = 0) vs. low (AA = 36, AC = 44, CC = 15) progranulin serum level. b Relative mRNA expression values distributed over the rs629301 genotype. c The corresponding DNA methylation levels (%) for PSRC1 assay 1. a is presented as number of individuals, and b and c are presented as bar plots showing mean ± SD values. ***P < 0.001
Discussion
Progranulin is a secreted protein with important functions in processes including immune and inflammatory responses, metabolism, and embryonic development [24]. It is assumed to be involved in chronic inflammation in obesity and T2D [1, 25]. Heritability of circulating progranulin levels is estimated to be around 30% [11]. A previous genome-wide association meta-analysis of five European cohorts along with subsequent eQTL analyses in peripheral blood mononuclear cells (PBMCs) pointed to PSRC1 as a potential target gene of the locus significantly associated with serum progranulin levels [11]. Moreover, functional studies in cell cultures supported the role of PSRC1 in the regulation of progranulin secretion. In particular, 60% reduction of PSRC1 expression by siRNA silencing in murine 3T3-L1 preadipocytes resulted in a consecutive reduction in progranulin secretion of approximately 30% [11]. To identify the potentially causal variant altering PSRC1 expression, we performed in silico and in vitro analyses and tested the effect of epigenetic regulation on progranulin serum levels in vivo. In summary, rs629301 turned out to be the most likely causative variant explaining the association of the abovementioned locus with circulating progranulin levels.
An initial PMCA prioritized four polymorphisms (rs12740374, rs629301, rs660240, rs7528419) potentially altering transcription factor binding sites (all with Sall > 7.0). The effects of these variants on transcriptional activity were tested by luciferase reporter assays, which revealed lower activities in vectors carrying the rs629301-A compared with the C allele. Moreover, EMSA indicated a different binding pattern for the two rs629301 alleles, with an additional prominent band for the A allele. Publicly available databases JASPAR and PROMO predicted a T allele-specific YY1 transcription factor binding site for this locus, which was subsequently confirmed by EMSA supershift using the respective YY1 antibody. Although these findings cannot explain the recently postulated role of PSRC1 in the control of progranulin secretion, they are definitely supporting the regulatory role of genetic variation in PSRC1 and, thus, are complementing the previously reported rs660240 as an eQTL for PSRC1 mRNA in PBMCs [11]. Furthermore, publicly available data for rs629301 additionally support the role of liver as target tissue for the identified effect on PSRC1 regulation by revealing the strongest eQTL on PSRC1 mRNA expression in liver (P < 1 × 10−33, Supplementary Table 2). In parallel, DNA promoter methylation of two regions (assay 1: 10CpGs; assay 2: 4CpGs) showed that subjects with high progranulin levels manifested a significantly higher mean DNA methylation in one promoter region (assay 1), which was in line with a significantly lower PSRC1 mRNA expression levels in blood. Consistently, rs629301-A allele was associated with lower PSRC1 mRNA expression and higher DNA methylation.
In summary, our data shed more light on the molecular mechanisms behind the associations of genetic variants with progranulin concentrations. Moreover, they strongly support PSRC1 as a plausible target gene of these genetic variants. Although the underlying functional mechanism linking progranulin and PSRC1 is not fully understood yet, there is a good evidence that PSRC1 might be involved in progranulin-dependent regulation of the Wnt/ß-catenin signaling pathway [26]. As has previously been shown, ß-catenin is directly regulated by PSRC1 [27]. An increased progranulin serum level may inhibit PSRC1 activity via Wnt binding and thereby lead to reduction of ß-catenin, further turning down ß-catenin-dependent transcription factors such as the TCF/LEF family [28]. Vice versa, PSRC1 might affect progranulin as shown by PSRC1 silencing experiments in vitro [11]. Whether there is a feedback allowing directional switches in mutual effects between PSRC1 and progranulin remains to be investigated in further studies. Nevertheless, further support for the relationship between PSRC1 and progranulin emerges from reports on progranulin-deficient mice [29] and patients with psoriasis, where progranulin was negatively correlated with ß-catenin expression in psoriatic skin lesions [30]. In addition, an enhanced PSCR1 activity may increase ß-catenin expression, which in turn may inhibit NF-kB expression and thereby lead to an anti-inflammatory potential as demonstrated in apoE−/− mice [31].
Conclusion
In conclusion, our data suggest that the progranulin-associated variant rs629301 modifies the transcription of PSRC1 through alteration of YY1 binding capacity. YY1 may act indirectly as progranulin repressor most likely by inhibiting PSRC1 expression. DNA methylation studies further support the role of PSRC1 in regulation of progranulin serum levels.
Abbreviations
- apoE:
-
apolipoprotein E
- CELSR2 :
-
cadherin EGF LAG seven-pass g-type receptor 2
- EMSA:
-
electrophoretic mobility shift assays
- eQTL:
-
expression quantitative trait loci
- GWAS:
-
genome-wide association study
- LD:
-
linkage disequilibrium
- MAF:
-
minor allele frequency
- MYBPHL :
-
myosin binding protein H like
- NF-kB:
-
nuclear factor kappa-light-chain-enhancer of activated B cells
- PBMC:
-
peripheral blood mononuclear cell
- PMCA:
-
phylogenetic module complexity analysis
- PSRC1 :
-
proline and serine rich coiled-coil 1
- PGRN/GRN :
-
progranulin
- SNP:
-
single-nucleotide polymorphism
- SORT1 :
-
sortilin 1
- TCF/LEF:
-
transcription factor/lymphoid enhancer binding factor
- YY1:
-
Yin Yang 1 transcription factor
References
Nguyen AD, Nguyen TA, Martens LH, Mitic LL, Farese RV Jr (2013) Progranulin: at the interface of neurodegenerative and metabolic diseases. Trends Endocrinol Metab 24(12):597–606
Youn B-S, Bang S-I, Kloting N, Park JW, Lee N, Oh J-E, Pi K-B, Lee TH, Ruschke K, Fasshauer M, Stumvoll M, Bluher M (2009) Serum progranulin concentrations may be associated with macrophage infiltration into omental adipose tissue. Diabetes 58(3):627–636
Matsubara T, Mita A, Minami K, Hosooka T, Kitazawa S, Takahashi K, Tamori Y, Yokoi N, Watanabe M, Matsuo E-I, Nishimura O, Seino S (2012) PGRN is a key adipokine mediating high fat diet-induced insulin resistance and obesity through IL-6 in adipose tissue. Cell Metab 15(1):38–50
Bhandari V, Bateman A (1992) Structure and chromosomal location of the human granulin gene. Biochem Biophys Res Commun 188(1):57–63
Ward ME, Chen R, Huang H-Y, Ludwig C, Telpoukhovskaia M, Taubes A, Boudin H, Minami SS, Reichert M, Albrecht P, Gelfand JM, Cruz-Herranz A, Cordano C, Alavi MV, Leslie S, Seeley WW, Miller BL, Bigio E, Mesulam MM, Bogyo MS, Mackenzie IR, Staropoli JF, Cotman SL, Huang EJ, Gan L, Green AJ (2017) Individuals with progranulin haploinsufficiency exhibit features of neuronal ceroid lipofuscinosis. Sci Transl Med 9(385):eaah5642
Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, Crook R, Melquist S, Kuntz K, Petersen R, Josephs K, Pickering-Brown SM, Graff-Radford N, Uitti R, Dickson D, Wszolek Z, Gonzalez J, Beach TG, Bigio E, Johnson N, Weintraub S, Mesulam M, White CL III, Woodruff B, Caselli R, Hsiung GY, Feldman H, Knopman D, Hutton M, Rademakers R (2006) Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet 15(20):2988–3001
Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, Snowden J, Adamson J, Sadovnick AD, Rollinson S, Cannon A, Dwosh E, Neary D, Melquist S, Richardson A, Dickson D, Berger Z, Eriksen J, Robinson T, Zehr C, Dickey CA, Crook R, McGowan E, Mann D, Boeve B, Feldman H, Hutton M (2006) Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442(7105):916–919
Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, Rademakers R, Vandenberghe R, Dermaut B, Martin J-J, van Duijn C, Peeters K, Sciot R, Santens P, de Pooter T, Mattheijssens M, van den Broeck M, Cuijt I, Vennekens K', de Deyn PP, Kumar-Singh S, van Broeckhoven C (2006) Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442(7105):920–924
Minami SS, Min S-W, Krabbe G, Wang C, Zhou Y, Asgarov R, Li Y, Martens LH, Elia LP, Ward ME, Mucke L, Farese RV Jr, Gan L (2014) Progranulin protects against amyloid beta deposition and toxicity in Alzheimer’s disease mouse models. Nat Med 20(10):1157–1164
Carrasquillo MM, Nicholson AM, Finch N, Gibbs JR, Baker M, Rutherford NJ, Hunter TA, DeJesus-Hernandez M, Bisceglio GD, Mackenzie IR, Singleton A, Cookson MR, Crook JE, Dillman A, Hernandez D, Petersen RC, Graff-Radford NR, Younkin SG, Rademakers R (2010) Genome-wide screen identifies rs646776 near sortilin as a regulator of progranulin levels in human plasma. Am J Hum Genet 87(6):890–897
Tönjes A, Scholz M, Krüger J, Krause K, Schleinitz D, Kirsten H, Gebhardt C, Marzi C, Grallert H, Ladenvall C, Heyne H, Laurila E, Kriebel J, Meisinger C, Rathmann W, Gieger C, Groop L, Prokopenko I, Isomaa B, Beutner F, Kratzsch J, Fischer-Rosinsky A, Pfeiffer A, Krohn K, Spranger J, Thiery J, Blüher M, Stumvoll M, Kovacs P (2018) Genome-wide meta-analysis identifies novel determinants of circulating serum progranulin. Hum Mol Genet 27(3):546–558
Nicholson AM, Finch NA, Almeida M, Perkerson RB, van Blitterswijk M, Wojtas A, Cenik B, Rotondo S, Inskeep V, Almasy L et al (2016) Prosaposin is a regulator of progranulin levels and oligomerization. Nat Commun 7:11992
Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ (2010) From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature 466(7307):714–719
Kjolby M, Andersen OM, Breiderhoff T, Fjorback AW, Pedersen KM, Madsen P, Jansen P, Heeren J, Willnow TE, Nykjaer A (2010) Sort1, encoded by the cardiovascular risk locus 1p13.3, is a regulator of hepatic lipoprotein export. Cell Metab 12(3):213–223
Cheng HS, Besla R, Li A, Chen Z, Shikatani EA, Nazari-Jahantigh M, Hammoutène A, Nguyen M-A, Geoffrion M, Cai L, Khyzha N, Li T, MacParland SA, Husain M, Cybulsky MI, Boulanger CM, Temel RE, Schober A, Rayner KJ, Robbins CS, Fish JE (2017) Paradoxical suppression of atherosclerosis in the absence of microRNA-146a. Circ Res 121(4):354–367
Goettsch C, Hutcheson JD, Aikawa M, Iwata H, Pham T, Nykjaer A, Kjolby M, Rogers M, Michel T, Shibasaki M, Hagita S, Kramann R, Rader DJ, Libby P, Singh SA, Aikawa E (2016) Sortilin mediates vascular calcification via its recruitment into extracellular vesicles. J Clin Invest 126(4):1323–1336
Zhang Z, Jiang W, Yang H, Lin Q, Qin X (2018) The miR-182/SORT1 axis regulates vascular smooth muscle cell calcification in vitro and in vivo. Exp Cell Res 362(2):324–331
Böttcher Y, Unbehauen H, Klöting N, Ruschke K, Körner A, Schleinitz D, Tönjes A, Enigk B, Wolf S, Dietrich K et al (2009) Adipose tissue expression and genetic variants of the bone morphogenetic protein receptor 1A gene (BMPR1A) are associated with human obesity. Diabetes 58(9):2119–2128
Veeramah KR, Tonjes A, Kovacs P, Gross A, Wegmann D, Geary P, Gasperikova D, Klimes I, Scholz M, Novembre J et al (2011) Genetic variation in the Sorbs of eastern Germany in the context of broader European genetic diversity. Eur J Human Genet 19(9):995–1001
Claussnitzer M, Dankel SN, Klocke B, Grallert H, Glunk V, Berulava T, Lee H, Oskolkov N, Fadista J, Ehlers K, Wahl S, Hoffmann C, Qian K, Rönn T, Riess H, Müller-Nurasyid M, Bretschneider N, Schroeder T, Skurk T, Horsthemke B, Spieler D, Klingenspor M, Seifert M, Kern MJ, Mejhert N, Dahlman I, Hansson O, Hauck SM, Blüher M, Arner P, Groop L, Illig T, Suhre K, Hsu YH, Mellgren G, Hauner H, Laumen H, Voight BF, Scott LJ, Steinthorsdottir V, Morris AP, Dina C, Welch RP, Zeggini E, Huth C, Aulchenko YS, Thorleifsson G, McCulloch LJ, Ferreira T, Grallert H, Amin N, Wu G, Willer CJ, Raychaudhuri S, McCarroll SA, Langenberg C, Hofmann OM, Dupuis J, Qi L, Segrè AV, van Hoek M, Navarro P, Ardlie K, Balkau B, Benediktsson R, Bennett AJ, Blagieva R, Boerwinkle E, Bonnycastle LL, Boström KB, Bravenboer B, Bumpstead S, Burtt NP, Charpentier G, Chines PS, Cornelis M, Couper DJ, Crawford G, Doney ASF, Elliott KS, Elliott AL, Erdos MR, Fox CS, Franklin CS, Ganser M, Gieger C, Grarup N, Green T, Griffin S, Groves CJ, Guiducci C, Hadjadj S, Hassanali N, Herder C, Isomaa B, Jackson AU, Johnson PRV, Jørgensen T, Kao WHL, Klopp N, Kong A, Kraft P, Kuusisto J, Lauritzen T, Li M, Lieverse A, Lindgren CM, Lyssenko V, Marre M, Meitinger T, Midthjell K, Morken MA, Narisu N, Nilsson P, Owen KR, Payne F, Perry JRB, Petersen AK, Platou C, Proença C, Prokopenko I, Rathmann W, Rayner NW, Robertson NR, Rocheleau G, Roden M, Sampson MJ, Saxena R, Shields BM, Shrader P, Sigurdsson G, Sparsø T, Strassburger K, Stringham HM, Sun Q, Swift AJ, Thorand B, Tichet J, Tuomi T, van Dam RM, van Haeften TW, van Herpt T, van Vliet-Ostaptchouk JV, Walters GB, Weedon MN, Wijmenga C, Witteman J, Bergman RN, Cauchi S, Collins FS, Gloyn AL, Gyllensten U, Hansen T, Hide WA, Hitman GA, Hofman A, Hunter DJ, Hveem K, Laakso M, Mohlke KL, Morris AD, Palmer CNA, Pramstaller PP, Rudan I, Sijbrands E, Stein LD, Tuomilehto J, Uitterlinden A, Walker M, Wareham NJ, Watanabe RM, Abecasis GR, Boehm BO, Campbell H, Daly MJ, Hattersley AT, Hu FB, Meigs JB, Pankow JS, Pedersen O, Wichmann HE, Barroso I, Florez JC, Frayling TM, Groop L, Sladek R, Thorsteinsdottir U, Wilson JF, Illig T, Froguel P, van Duijn CM, Stefansson K, Altshuler D, Boehnke M, McCarthy MI (2014) Leveraging cross-species transcription factor binding site patterns: from diabetes risk loci to disease mechanisms. Cell 156(1–2):343–358
Rohde K, Keller M, Klos M, Schleinitz D, Dietrich A, Schon MR, Gartner D, Lohmann T, Dressler M, Stumvoll M et al (2014) Adipose tissue depot specific promoter methylation of TMEM18. J Mol Med 92(8):881–888
Tonjes A, Koriath M, Schleinitz D, Dietrich K, Bottcher Y, Rayner NW, Almgren P, Enigk B, Richter O, Rohm S et al (2009) Genetic variation in GPR133 is associated with height: genome wide association study in the self-contained population of Sorbs. Hum Mol Genet 18(23):4662–4668
R Development Core Team (2008) (2008) R. A language and environment for statistical computing. R Foundation for Statistical Computing, Wien
Tolkatchev D, Malik S, Vinogradova A, Wang P, Chen Z, Xu P, Bennett HPJ, Bateman A, Ni F (2008) Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities. Protein Sci 17(4):711–724
Cenik B, Sephton CF, Kutluk Cenik B, Herz J, Yu G (2012) Progranulin: a proteolytically processed protein at the crossroads of inflammation and neurodegeneration. J Biol Chem 287(39):32298–32306
Fu Y, Sun Y, Zhou M, Wang X, Wang Z, Wei X, Zhang Y, Su Z, Liang K, Tang W et al (2017) Therapeutic potential of progranulin in hyperhomocysteinemia-induced cardiorenal dysfunction. Hypertension 69(2):259–266
Hsieh P-C, Chiang M-L, Chang J-C, Yan Y-T, Wang F-F, Chou Y-C (2012) DDA3 stabilizes microtubules and suppresses neurite formation. J Cell Sci 125(17):4171
Chen N, Wang J (2018) Wnt/β-catenin signaling and obesity. Front Physiol 9:792
Zhao Y-P, Liu B, Tian Q-Y, Wei J-L, Richbourgh B, Liu C-J (2015) Progranulin protects against osteoarthritis through interacting with TNF-α and β-catenin signalling. Ann Rheum Dis 74(12):2244–2253
Farag AGA, Shoaib MA, Samaka RM, Abdou AG, Mandour MM, Ibrahim RAL (2019) Progranulin and beta-catenin in psoriasis: an immunohistochemical study. J Cosmet Dermatol 18:2019–2026
Guo K, Hu L, Xi D, Zhao J, Liu J, Luo T, Ma Y, Lai W, Guo Z (2018) PSRC1 overexpression attenuates atherosclerosis progression in apoE-/- mice by modulating cholesterol transportation and inflammation. J Mol Cell Cardiol 116:69–80
Acknowledgments
Open Access funding provided by Projekt DEAL. We thank all those who participated in the studies. We would like to acknowledge statistical support by Tobias Wohland and excellent technical assistance by Beate Gutsmann and Ines Müller.
Funding
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation – Projektnummer 209933838 – SFB 1052; B03, C01; SPP 1629 TO 718/2- 1, and GZ: KE 2182/1-1), from the German Diabetes Association and from the DHFD (Diabetes Hilfs- und Forschungsfonds Deutschland). IFB Adiposity Diseases is supported by the Federal Ministry of Education and Research (BMBF), Germany, FKZ: 01EO1501 (AD2-060E, AD2-06E95, AD2-7123, AD2-7118, K7-117, AD2-6E96, AD2-6E97).
Author information
Authors and Affiliations
Contributions
MK and CG performed most laboratory work, data analysis, and statistical work. MK and PK wrote the first manuscript draft. SH prepared luciferase constructs and performed luciferase assays. HH supported the PMCA interpretation. MSc, YB, and MS supported the critical data interpretation and reviewed the manuscript. MS and AT are PIs of the Sorbs cohort. AT and PK initiated, conceived, and designed the study. MK, AT, and PK contributed to critical data discussion and wrote the final version of the manuscript.
Corresponding authors
Ethics declarations
All participants gave their written informed consent, and the study was approved by the ethics committee of the University of Leipzig.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(XLSX 20.2 kb)s
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Keller, M., Gebhardt, C., Huth, S. et al. Genetically programmed changes in transcription of the novel progranulin regulator. J Mol Med 98, 1139–1148 (2020). https://doi.org/10.1007/s00109-020-01942-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01942-7