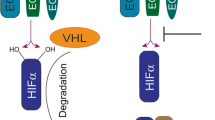
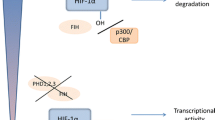
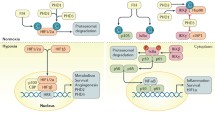
Abstract
The intestinal mucosa provides a selective barrier between the anaerobic lumen and a highly metabolic lamina propria. A number of recent studies indicate that acute inflammation of the mucosa can result in tissue hypoxia and associated shifts in tissue metabolism. The activation of hypoxia-inducible factor (HIF) under these conditions has been demonstrated to function as an endogenous molecular cue to promote resolution of inflammation, particularly through the orchestration of barrier repair toward homeostasis. Given the central role of oxygen in tissue metabolism, ongoing studies have defined metabolic endpoints of HIF stabilization as important biomarkers of disease activity. Such findings make HIF and HIF-associated metabolic pathways particularly attractive therapeutic targets in inflammatory bowel disease (IBD). Here, we review the recent literature related to tissue metabolism in IBD.



Similar content being viewed by others
References
Khor B, Gardet A, Xavier RJ (2011) Genetics and pathogenesis of inflammatory bowel disease. Nature 474:307–317
Laukoetter MG, Nava P, Nusrat A (2008) Role of the intestinal barrier in inflammatory bowel disease. World J Gastroenterol 14:401–407
Turner JR (2009) Intestinal mucosal barrier function in health and disease. Nat Rev Immunol 9:799–809
Colgan SP, Taylor CT (2010) Hypoxia: an alarm signal during intestinal inflammation. Nat Rev Gastroenterol Hepatol 7:281–287
Glover LE, Lee JS, Colgan SP (2016) Oxygen metabolism and barrier regulation in the intestinal mucosa. J Clin Invest 126:3680–3688
Zheng L, Kelly CJ, Colgan SP (2015) Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A review in the theme: cellular responses to hypoxia. Am J Physiol Cell Physiol 309:C350–C360
Colgan SP, Campbell EL, Kominsky DJ (2016) Hypoxia and mucosal inflammation. Ann Rev Pathol 11:77–100
Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI (1986) Increased intestinal permeability in patients with Crohn’s disease and their relatives. A possible etiologic factor. Ann Intern Med 105:883–885
Madsen K, Cornish A, Soper P, McKaigney C, Jijon H, Yachimec C, Doyle J, Jewell L, De Simone C (2001) Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591
Ratcliffe PJ (2007) HIF-1 and HIF-2: working alone or together in hypoxia? J Clin Invest 117:862–865
Schofield CJ, Ratcliffe PJ (2004) Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5:343–354
Wenger RH, Stiehl DP, Camenisch G (2005) Integration of oxygen signaling at the consensus HRE. Sci STKE 2005:re12
Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C (2009) HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest 119:1159–1166
Furuta GT, Turner JR, Taylor CT, Hershberg RM, Comerford K, Narravula S, Podolsky DK, Colgan SP (2001) Hypoxia-inducible factor 1-dependent induction of intestinal trefoil factor protects barrier function during hypoxia. J Exp Med 193:1027–1034
Simpson RJ, McKie AT (2009) Regulation of intestinal iron absorption: the mucosa takes control? Cell Metab 10:84–87
Stein J, Hartmann F, Dignass AU Diagnosis and management of iron deficiency anemia in patients with IBD. Nat Rev Gastroenterol Hepatol
Taylor CT, Colgan SP (2007) Hypoxia and gastrointestinal disease. J Mol Med 85:1295–1300
Fox CJ, Hammerman PS, Thompson CB (2005) Fuel feeds function: energy metabolism and the T-cell response. Nat Rev Immunol 5:844–852
Kominsky DJ, Campbell EL, Colgan SP (2010) Metabolic shifts in immunity and inflammation. J Immunol 184:4062–4068
Lewis JS, Lee JA, Underwood JC, Harris AL, Lewis CE (1999) Macrophage responses to hypoxia: relevance to disease mechanisms. J Leukoc Biol 66:889–900
El-Benna J, Dang PM, Gougerot-Pocidalo MA (2008) Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane. Semin Immunopathol 30:279–289
Gabig TG, Bearman SI, Babior BM (1979) Effects of oxygen tension and pH on the respiratory burst of human neutrophils. Blood 53:1133–1139
Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, Bowers BE, Bayless AJ, Scully M, Saeedi BJ, Golden-Mason L et al (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40:66–77
Bai A, Moss A, Rothweiler S, Longhi MS, Wu Y, Junger WG, Robson SC (2015) NADH oxidase-dependent CD39 expression by CD8(+) T cells modulates interferon gamma responses via generation of adenosine. Nat Commun 6: 10.1038/ncomms9819.
Huang JS, Noack D, Rae J, Ellis BA, Newbury R, Pong AL, Lavine JE, Curnutte JT, Bastian J (2004) Chronic granulomatous disease caused by a deficiency in p47(phox) mimicking Crohn’s disease. Clin Gastroenterol Hepatol 2:690–695
Werlin SL, Chusid MJ, Caya J, Oechler HW (1982) Colitis in chronic granulomatous disease. Gastroenterology 82:328–331
Giatromanolaki A, Sivridis E, Maltezos E, Papazoglou D, Simopoulos C, Gatter KC, Harris AL, Koukourakis MI (2003) Hypoxia inducible factor 1alpha and 2alpha overexpression in inflammatory bowel disease. J Clin Pathol 56:209–213
Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL (1996) Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol 16:4604–4613
Colgan SP, Fennimore B, Ehrentraut SF (2013) Adenosine and gastrointestinal inflammation. J Mol Med (Berl) 91:157–164
Colgan SP, Eltzschig HK (2012) Adenosine and hypoxia-inducible factor signaling in intestinal injury and recovery. Annu Rev Physiol 74:153–175
Eltzschig HK, Eckle T, Mager A, Küper N, Karcher C, Weissmüller T, Boengler K, Schulz R, Robson SC, Colgan SP (2006) ATP release from activated neutrophils occurs via Connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res 99:1100–1108
Neves AR, Castelo-Branco MTL, Figliuolo VR, Bernardazzi C, Buongusto F, Yoshimoto A, Nanini HF, Coutinho CMLM, Carneiro AJV, Coutinho-Silva R et al (2014) Overexpression of ATP-activated P2X7 receptors in the intestinal mucosa is implicated in the pathogenesis of Crohn’s disease. Inflamm Bowel Dis 20:444–457
Eltzschig HK, Sitkovsky MV, Robson SC (2012) Purinergic signaling during inflammation. N Engl J Med 367:2322–2333
Strohmeier GR, Lencer WI, Patapoff TW, Thompson LF, Carlson SL, Moe SJ, Carnes DK, Mrsny RJ, Madara JL (1997) Surface expression, polarization, and functional significance of CD73 in human intestinal epithelia. J Clin Investig 99:2588–2601
Thompson LF, Eltzschig HK, Ibla JC, Van De Wiele CJ, Resta R, Morote-Garcia JC, Colgan SP (2004) Crucial role for ecto-5′-nucleotidase (CD73) in vascular leakage during hypoxia. J Exp Med 200:1395–1405
Weissmüller T, Campbell EL, Rosenberger P, Scully M, Beck PL, Furuta GT, Colgan SP (2008) PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell–expressed ecto-NTPDases. J Clin Investig 118:3682–3692
Bai A, Moss A, Kokkotou E, Usheva A, Sun X, Cheifetz A, Zheng Y, Longhi MS, Gao W, Wu Y et al (2014) CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol (Baltimore, Md: 1950) 193:3366–3377
Friedman DJ, Künzli BM, A-Rahim YI, Sevigny J, Berberat PO, Enjyoji K, Csizmadia E, Friess H, Robson SC (2009) CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci 106:16788–16793
Bynoe MS, Waickman AT, Mahamed DA, Mueller C, Mills JH, Czopik A (2012) CD73 is critical for the resolution of murine colonic inflammation. BioMed Res Inte 2012
Louis NA, Robinson AM, Macmanus CF, Karhausen J, Scully M, Colgan SP (2008) Control of IFN-{alpha}a by CD73: implications for mucosal inflammation. J Immunol 180:4246–4255
Rybaczyk L, Rozmiarek A, Circle K, Grants I, Needleman B, Wunderlich JE, Huang K, Christofi FL (2009) New bioinformatics approach to analyze gene expressions and signaling pathways reveals unique purine gene dysregulation profiles that distinguish between CD and UC. Inflamm Bowel Dis 15:971–984
Colgan SP, Fennimore B, Ehrentraut SF (2013) Adenosine and gastrointestinal inflammation. J Mol Med 91:157–164
Aherne C, Saeedi B, Collins C, Masterson J, McNamee E, Perrenoud L, Rapp C, Curtis V, Bayless A, Fletcher A et al (2015) Epithelial-specific A2B adenosine receptor signaling protects the colonic epithelial barrier during acute colitis. Mucosal Immunol 8:1324–1338
Lawrence DW, Comerford KM, Colgan SP (2002) Role of VASP in reestablishment of epithelial tight junction assembly after Ca2+ switch. Am J Physiol Cell Physiol 282:C1235–C1245
Strohmeier GR, Reppert SM, Lencer WI, Madara JL (1995) The a adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J Biol Chem 270:2387–2394
Amir RE, Iwai K, Ciechanover A (2002) The NEDD8 pathway is essential for SCFβ-TrCP-mediated ubiquitination and processing of the NF-κB precursor p105. J Biol Chem 277:23253–23259
Sitkovsky M, Lukashev D (2005) Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol 5:712–721
Sitkovsky M, Lukashev D, Deaglio S, Dwyer K, Robson SC, Ohta A (2008) Adenosine A2A receptor antagonists: blockade of adenosinergic effects and T regulatory cells. Br J Pharmacol 153:S457–S464
Sitkovsky MV (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 30:102–108
Kusu T, Kayama H, Kinoshita M, Jeon SG, Ueda Y, Goto Y, Okumura R, Saiga H, Kurakawa T, Ikeda K et al (2013) Ecto-nucleoside triphosphate diphosphohydrolase 7 controls Th17 cell responses through regulation of luminal ATP in the small intestine. J Immunol 190:774–783
Naganuma M, Wiznerowicz EB, Lappas CM, Linden J, Worthington MT, Ernst PB (2006) Cutting edge: critical role for A2A adenosine receptors in the T cell-mediated regulation of colitis. J Immunol 177:2765–2769
Kurtz CC, Drygiannakis I, Naganuma M, Feldman S, Bekiaris V, Linden J, Ware CF, Ernst PB (2014) Extracellular adenosine regulates colitis through effects on lymphoid and nonlymphoid cells. Am J Physiol Gastrointest Liver Physiol 307:G338–G346
Sainio EL, Pulkki K, Young SN (1996) L-tryptophan: biochemical, nutritional and pharmacological aspects. Amino Acids 10:21–47
Matteoli G, Mazzini E, Iliev ID, Mileti E, Fallarino F, Puccetti P, Chieppa M, Rescigno M (2010) Gut CD103+ dendritic cells express indoleamine 2,3-dioxygenase which influences T regulatory/T effector cell balance and oral tolerance induction. Gut 59:595–604
Nguyen NT, Kimura A, Nakahama T, Chinen I, Masuda K, Nohara K, Fujii-Kuriyama Y, Kishimoto T (2010) Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A 107:19961–19966
Pallotta MT, Orabona C, Volpi C, Vacca C, Belladonna ML, Bianchi R, Servillo G, Brunacci C, Calvitti M, Bicciato S et al (2011) Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 12:870–878
Ciorba MA, Bettonville EE, McDonald KG, Metz R, Prendergast GC, Newberry RD, Stenson WF (2010) Induction of IDO-1 by immunostimulatory DNA limits severity of experimental colitis. J Immunol 184:3907–3916
Muzaki AR, Tetlak P, Sheng J, Loh SC, Setiagani YA, Poidinger M, Zolezzi F, Karjalainen K, Ruedl C (2015) Intestinal CD103CD11b dendritic cells restrain colitis via IFN-gamma-induced anti-inflammatory response in epithelial cells. Mucosal Immunol. doi:10.1038/mi.2015.64
Takamatsu M, Hirata A, Ohtaki H, Hoshi M, Hatano Y, Tomita H, Kuno T, Saito K, Hara A (2013) IDO1 plays an immunosuppressive role in 2,4,6-trinitrobenzene sulfate-induced colitis in mice. J Immunol 1 91:3057–3064
Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF (2003) Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology 125:1762–1773
Shon WJ, Lee YK, Shin JH, Choi EY, Shin DM (2015) Severity of DSS-induced colitis is reduced in Ido1-deficient mice with down-regulation of TLR-MyD88-NF-kB transcriptional networks. Sci Rep 5:17305
Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, Shi HN, Cherayil BJ (2008) Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect Immun 76:3045–3053
Opitz CA, Litzenburger UM, Sahm F, Ott M, Tritschler I, Trump S, Schumacher T, Jestaedt L, Schrenk D, Weller M et al (2011) An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 478:197–203
Bessede A, Gargaro M, Pallotta MT, Matino D, Servillo G, Brunacci C, Bicciato S, Mazza EM, Macchiarulo A, Vacca C et al (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511:184–190
Flaveny CA, Murray IA, Chiaro CR, Perdew GH (2009) Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol Pharmacol 75:1412–1420
Bacsi SG, Reisz-Porszasz S, Hankinson O (1995) Orientation of the heterodimeric aryl hydrocarbon (dioxin) receptor complex on its asymmetric DNA recognition sequence. Mol Pharmacol 47:432–438
Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ (1996) Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol 140:173–179
Gielen JE, Goujon FM, Nebert DW (1972) Genetic regulation of aryl hydrocarbon hydroxylase induction. II. Simple Mendelian expression in mouse tissues in vivo. J Biol Chem 247:1125–1137
Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol 185:3190–3198
Mascanfroni ID, Takenaka MC, Yeste A, Patel B, Wu Y, Kenison JE, Siddiqui S, Basso AS, Otterbein LE, Pardoll DM et al (2015) Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat Med 21:638–646
Ji T, Xu C, Sun L, Yu M, Peng K, Qiu Y, Xiao W, Yang H (2015) Aryl hydrocarbon receptor activation down-regulates IL-7 and reduces inflammation in a mouse model of DSS-induced colitis. Dig Dis Sci 60:1958–1966
Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL (2008) Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71
Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109
Duarte JH, Di Meglio P, Hirota K, Ahlfors H, Stockinger B (2013) Differential influences of the aryl hydrocarbon receptor on Th17 mediated responses in vitro and in vivo. PLoS One 8:e79819
Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS (2011) Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS One 6:e23522
Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limon P, Paiva RS, Ching T et al (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523:221–225
Lanis JM, Alexeev EE, Curtis VF, Kitzenberg DA, Kao DJ, Battista KD, Gerich ME, Glover LE, Kominsky DJ, Colgan SP (2017) Tryptophan metabolite activation of the aryl hydrocarbon receptor regulates IL-10 receptor expression on intestinal epithelia. Mucosal Immunol. doi:10.1038/mi.2016.133
Kominsky DJ, Campbell EL, Ehrentraut SF, Wilson KE, Kelly CJ, Glover LE, Collins CB, Bayless AJ, Saeedi B, Dobrinskikh E et al (2014) IFN-gamma-mediated induction of an apical IL-10 receptor on polarized intestinal epithelia. J Immunol 192:1267–1276
Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230
Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y, Tome D (2013) Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 68:95–107
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8
Herrmann G, Jayamani E, Mai G, Buckel W (2008) Energy conservation via electron-transferring flavoprotein in anaerobic bacteria. J Bacteriol 190:784–791
Louis P, Flint HJ (2007) Development of a semiquantitative degenerate real-time pcr-based assay for estimation of numbers of butyryl-coenzyme a (CoA) CoA transferase genes in complex bacterial samples. Appl Environ Microbiol 73:2009–2012
Vital M, Penton CR, Wang Q, Young VB, Antonopoulos DA, Sogin ML, Morrison HG, Raffals L, Chang EB, Huffnagle GB et al (2013) A gene-targeted approach to investigate the intestinal butyrate-producing bacterial community. Microbiome 1:1–8
Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ (2008) Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 27:104–119
Miquel S, Martin R, Rossi O, Bermudez-Humaran LG, Chatel JM, Sokol H, Thomas M, Wells JM, Langella P (2013) Faecalibacterium prausnitzii and human intestinal health. Curr Opin Microbiol 16:255–261
Kelly CJ, Zheng L, Campbell EL, Saeedi B, Scholz CC, Bayless AJ, Wilson KE, Glover LE, Kominsky DJ, Magnuson A et al (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17:662–671
Berry D, Reinisch W (2013) Intestinal microbiota: a source of novel biomarkers in inflammatory bowel diseases? Best Pract Res Clin Gastroenterol 27:47–58
Jin UH, Lee SO, Sridharan G, Lee K, Davidson LA, Jayaraman A, Chapkin RS, Alaniz R, Safe S (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol Pharmacol 85:777–788
Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F et al (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385
Bansal T, Alaniz RC, Wood TK, Jayaraman A (2010) The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc Natl Acad Sci U S A 107:228–233
Eltzschig HK, Bratton DL, Colgan SP (2014) Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov 13:852–869
Acknowledgements
This work was supported by NIH grants DK50189 / DK104713 / DK095491 and VA Merit Award 1I01BX002182.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lanis, J.M., Kao, D.J., Alexeev, E.E. et al. Tissue metabolism and the inflammatory bowel diseases. J Mol Med 95, 905–913 (2017). https://doi.org/10.1007/s00109-017-1544-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-017-1544-2