Abstract
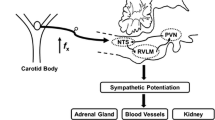
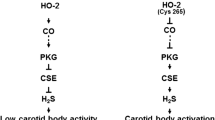
Systemic hypertension is one of the most prevalent cardiovascular diseases. Sleep-disordered breathing (SDB) with recurrent apnea is a major risk factor for developing essential hypertension. Chronic intermittent hypoxia (CIH) is a hallmark manifestation of recurrent apnea. Rodent models patterned after the O2 profiles seen with SDB patients showed that CIH is the major stimulus for causing systemic hypertension. This article reviews the physiological and molecular basis of CIH-induced hypertension. Physiological studies have identified that augmented carotid body chemosensory reflex and the resulting increase in sympathetic nerve activity are major contributors to CIH-induced hypertension. Analysis of molecular mechanisms revealed that CIH activates hypoxia-inducible factor (HIF)-1 and suppresses HIF-2-mediated transcription. Dysregulation of HIF-1- and HIF-2-mediated transcription leads to imbalance of pro-oxidant and anti-oxidant enzyme gene expression resulting in increased reactive oxygen species (ROS) generation in the chemosensory reflex which is central for developing hypertension.


Similar content being viewed by others
References
Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J (2005) Global burden of hypertension: analysis of worldwide data. Lancet 365:217–223
Carretero OA, Oparil S (2000) Essential hypertension. Part I: definition and etiology. Circulation 101:329–335
Hla KM, Young TB, Bidwell T, Palta M, Skatrud JB, Dempsey J (1994) Sleep apnea and hypertension. A population-based study. Ann Intern Med 120:382–388
Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D'Agostino RB, Newman AB, Lebowitz MD, Pickering TG (2000) Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study JAMA 283:1829–1836
Garcia-Rio F, Racionero MA, Pino JM, Martinez I, Ortuno F, Villasante C, Villamor J (2000) Sleep apnea and hypertension. Chest 117:1417–1425
Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Javier Nieto F, O'Connor GT, Boland LL, Schwartz JE, Samet JM (2001) Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the sleep heart health study. Am J Respir Crit Care Med 163:19–25
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S (1993) The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 328:1230–1235
Peppard PE, Young T, Palta M, Skatrud J (2000) Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 342:1378–1384
Fletcher EC (1995) An animal model of the relationship between systemic hypertension and repetitive episodic hypoxia as seen in sleep apnoea. J Sleep Res 4:71–77
Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, Prabhakar NR (2006) Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol 575:229–239
Peng YJ, Yuan G, Khan S, Nanduri J, Makarenko VV, Reddy VD, Vasavda C, Kumar GK, Semenza GL, Prabhakar NR (2014) Regulation of hypoxia-inducible factor-α isoforms and redox state by carotid body neural activity in rats. J Physiol 592:3841–3858
Prabhakar NR, Semenza GL (2012) Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev 92:967–1003
Semenza GL (2014) Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 9:47–71
Narkiewicz K, Somers VK (1997) The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 15:1613–1619
Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG (1993) Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest 103:1763–1768
Fletcher EC, Miller J, Schaaf JW, Fletcher JG (1987) Urinary catecholamines before and after tracheostomy in patients with obstructive sleep apnea and hypertension. Sleep 10:35–44
Marrone O, Riccobono L, Salvaggio A, Mirabella A, Bonanno A, Bonsignore MR (1993) Catecholamines and blood pressure in obstructive sleep apnea syndrome. Chest 103:722–727
Somers VK, Dyken ME, Clary MP, Abboud FM (1995) Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96:1897–1904
Becker HF, Jerrentrup A, Ploch T, Grote L, Penzel T, Sullivan CE, Peter JH (2003) Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation 107:68–73
Imadojemu VA, Mawji Z, Kunselman A, Gray KS, Hogeman CS, Leuenberger UA (2007) Sympathetic chemoreflex responses in obstructive sleep apnea and effects of continuous positive airway pressure therapy. Chest 131:1406–1413
Greenberg HE, Sica A, Batson D, Scharf SM (1999) Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol 86:298–305
Huang J, Lusina S, Xie T, Ji E, Xiang S, Liu Y, Weiss JW (2009) Sympathetic response to chemostimulation in conscious rats exposed to chronic intermittent hypoxia. Respir Physiol Neurobiol 166:102–106
Dick TE, Hsieh YH, Wang N, Prabhakar N (2007) Acute intermittent hypoxia increases both phrenic and sympathetic nerve activities in the rat. Exp Physiol 92:87–97
Marcus NJ, Li YL, Bird CE, Schultz HD, Morgan BJ (2010) Chronic intermittent hypoxia augments chemoreflex control of sympathetic activity: role of the angiotensin II type 1 receptor. Respir Physiol Neurobiol 171:36–45
Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH (2008) Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol 586:3253–3265
Fletcher EC, Lesske J, Culman J, Miller CC, Unger T (1992) Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20:612–619
Kumar P, Prabhakar NR (2012) Peripheral chemoreceptors: function and plasticity of the carotid body. Comprehensive Physiol 2:141–219
Hedner JA, Wilcox I, Laks L, Grunstein RR, Sullivan CE (1992) A specific and potent pressor effect of hypoxia in patients with sleep apnea. Am Rev Respir Dis 146:1240–1245
Kara T, Narkiewicz K, Somers VK (2003) Chemoreflexes—physiology and clinical implications. Acta Physiol Scand 177:377–384
Narkiewicz K, van de Borne PJ, Pesek CA, Dyken ME, Montano N, Somers VK (1999) Selective potentiation of peripheral chemoreflex sensitivity in obstructive sleep apnea. Circulation 99:1183–1189
Tafil-Klawe M, Thiele AE, Raschke F, Mayer J, Peter JH, von Wichert W (1991) Peripheral chemoreceptor reflex in obstructive sleep apnea patients; a relationship between ventilatory response to hypoxia and nocturnal bradycardia during apnea events. Pneumologie 45(Suppl 1):309–311
Rey S, Del Rio R, Alcayaga J, Iturriaga R (2004) Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560:577–586
Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR (2006) Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol 577:705–716
Braga VA, Soriano RN, Machado BH (2006) Sympathoexcitatory response to peripheral chemoreflex activation is enhanced in juvenile rats exposed to chronic intermittent hypoxia. Exp Physiol 91:1025–1031
Honda Y (1985) Role of carotid chemoreceptors in control of breathing at rest and in exercise: studies on human subjects with bilateral carotid body resection. Jpn J Physiol 35:535–544
Somers VK, Abboud FM (1993) Chemoreflexes—responses, interactions and implications for sleep apnea. Sleep 16:S30–S33, discussion S33-34
Fletcher EC, Lesske J, Behm R, Miller CC 3rd, Stauss H, Unger T (1992) Carotid chemoreceptors, systemic blood pressure, and chronic episodic hypoxia mimicking sleep apnea. J Appl Physiol 72:1978–1984
Peng YJ, Prabhakar NR (2004) Effect of two paradigms of chronic intermittent hypoxia on carotid body sensory activity. J Appl Physiol 96:1236–1242, discussion 1196
Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR (2003) Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A 100:10073–10078
Del Rio R, Munoz C, Arias P, Court FA, Moya EA, Iturriaga R (2011) Chronic intermittent hypoxia-induced vascular enlargement and VEGF upregulation in the rat carotid body is not prevented by antioxidant treatment. Am J Physiol Lung Cell Mol Physiol 301:L702–L711
Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA (1997) Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest 99:106–109
Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P (2011) 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37:119–128
Prabhakar NR (2013) Sensing hypoxia: physiology, genetics and epigenetics. J Physiol 591:2245–2257
Kline DD, Ramirez-Navarro A, Kunze DL (2007) Adaptive depression in synaptic transmission in the nucleus of the solitary tract after in vivo chronic intermittent hypoxia: evidence for homeostatic plasticity. J Neurosci 27:4663–4673
Sica AL, Greenberg HE, Ruggiero DA, Scharf SM (2000) Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121:173–184
Silva AQ, Schreihofer AM (2011) Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol 589:1463–1476
Bao G, Metreveli N, Li R, Taylor A, Fletcher EC (1997) Blood pressure response to chronic episodic hypoxia: role of the sympathetic nervous system. J Appl Physiol 83:95–101
Roux JC, Brismar H, Aperia A, Lagercrantz H (2005) Developmental changes in HIF transcription factor in carotid body: relevance for O2 sensing by chemoreceptors. Pediatr Res 58:53–57
Lam SY, Tipoe GL, Liong EC, Fung ML (2008) Differential expressions and roles of hypoxia-inducible factor-1alpha, -2alpha and -3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol 23:271–280
Lam SY, Tipoe GL, Liong EC, Fung ML (2006) Hypoxia-inducible factor (HIF)-1alpha and endothelin-1 expression in the rat carotid body during intermittent hypoxia. Adv Exp Med Biol 580:21–27, discussion 351-359
Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR (2009) Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci U S A 106:1199–1204
Barnett S, Mulligan E, Wagerle LC, Lahiri S (1988) Measurement of carotid body blood flow in cats by use of radioactive microspheres. J Appl Physiol 65:2484–2489
Clarke JA, de Burgh DM, Ead HW (1986) Dimensions and volume of the carotid body in the adult cat, and their relation to the specific blood flow through the organ. A histological and morphometric study. Acta Anat (Basel) 126:84–86
de Burgh Daly DM, Lambertsen CJ, Schweitzer A (1954) Observations on the volume of blood flow and oxygen utilization of the carotid body in the cat. J Physiol 125:67–89
Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR (2013) Xanthine oxidase mediates hypoxia-inducible factor-2alpha degradation by intermittent hypoxia. PLoS One 8:e75838
Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR (2008) Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol 217:674–685
Nanduri J, Vaddi D, Khan S, Wang N, Makarenko V, Semenza G, Prabhakar NR (2015) HIF-1α activation by intermittent hypoxia requires NADPH oxidase stimulation by xanthine oxidase. PLos One: 10:e0119762
Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR (2005) Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem 280:4321–4328
Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR (2011) Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol 226:2925–2933
Thompson RJ, Jackson A, Nurse CA (1997) Developmental loss of hypoxic chemosensitivity in rat adrenomedullary chromaffin cells. J Physiol 498(Pt 2):503–510
Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY et al (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 12:149–162
Yu AY, Shimoda LA, Iyer NV, Huso DL, Sun X, McWilliams R, Beaty T, Sham JS, Wiener CM, Sylvester JT et al (1999) Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest 103:691–696
Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA et al (2003) Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet 35:331–340
Peng YJ, Nanduri J, Khan SA, Yuan G, Wang N, Kinsman B, Vaddi DR, Kumar GK, Garcia JA, Semenza GL et al (2011) Hypoxia-inducible factor 2alpha (HIF-2alpha) heterozygous-null mice exhibit exaggerated carotid body sensitivity to hypoxia, breathing instability, and hypertension. Proc Natl Acad Sci U S A 108:3065–3070
Yuan G, Peng YJ, Reddy VD, Makarenko VV, Nanduri J, Khan SA, Garcia JA, Kumar GK, Semenza GL, Prabhakar NR (2013) Mutual antagonism between hypoxia-inducible factors 1α and 2α regulates oxygen sensing and cardio-respiratory homeostasis. Proc Natl Acad Sci U S A 110:E1788–E1796
Prabhakar NR (2001) Oxygen sensing during intermittent hypoxia: cellular and molecular mechanisms. J Appl Physiol 90:1986–1994
Dyugovskaya L, Lavie P, Lavie L (2002) Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med 165:934–939
Prabhakar NR, Kumar GK, Nanduri J, Semenza GL (2007) ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal 9:1397–1403
Grebe M, Eisele HJ, Weissmann N, Schaefer C, Tillmanns H, Seeger W, Schulz R (2006) Antioxidant vitamin C improves endothelial function in obstructive sleep apnea. Am J Respir Crit Care Med 173:897–901
Gardner PR (2002) Aconitase: sensitive target and measure of superoxide. Methods Enzymol 349:9–23
Peng YJ, Nanduri J, Raghuraman G, Wang N, Kumar GK, Prabhakar NR (2013) Role of oxidative stress-induced endothelin-converting enzyme activity in the alteration of carotid body function by chronic intermittent hypoxia. Exp Physiol 98:1620–1630
Peng YJ, Nanduri J, Yuan G, Wang N, Deneris E, Pendyala S, Natarajan V, Kumar GK, Prabhakar NR (2009) NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci 29:4903–4910
Ortiz FC, Del Rio R, Varas R, Iturriaga R (2012) Contribution of TASK-like potassium channels to the enhanced rat carotid body responsiveness to hypoxia. Adv Exp Med Biol 758:365–371
Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR (2010) NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci 30:10763–10772
Pawar A, Nanduri J, Yuan G, Khan SA, Wang N, Kumar GK, Prabhakar NR (2009) Reactive oxygen species-dependent endothelin signaling is required for augmented hypoxic sensory response of the neonatal carotid body by intermittent hypoxia. Am J Physiol Regul Integr Comp Physiol 296:R735–R742
Bedard K, Krause KH (2007) The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87:245–313
Ambrosio G, Zweier JL, Duilio C, Kuppusamy P, Santoro G, Elia PP, Tritto I, Cirillo P, Condorelli M, Chiariello M (1993) Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J Biol Chem 268:18532–18541
Khan SA, Nanduri J, Yuan G, Kinsman B, Kumar GK, Joseph J, Kalyanaraman B, Prabhakar NR (2011) NADPH oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: relevance to blood pressure changes in rats. Antioxid Redox Signal 14:533–542
Shimoda LA, Laurie SS (2014) HIF and pulmonary vascular responses to hypoxia. J Appl Physiol 116:867–874
Acknowledgments
Research from authors’ laboratory is supported by grants from the National Institutes of Health, Heart, Lung, and Blood Institute PO1-HL-90554 and UH2-HL-123610.
Author statement
The authors hereby state that the contents of this review article have not been submitted for publication to any other journal.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nanduri, J., Peng, YJ., Yuan, G. et al. Hypoxia-inducible factors and hypertension: lessons from sleep apnea syndrome. J Mol Med 93, 473–480 (2015). https://doi.org/10.1007/s00109-015-1274-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-015-1274-2