Abstract
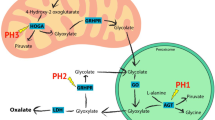
Perturbations in glyoxylate metabolism lead to the accumulation of oxalate and give rise to primary hyperoxalurias, recessive disorders characterized by kidney stone disease. Loss-of-function mutations in HOGA1 (formerly DHDPSL) are responsible for primary hyperoxaluria type III. HOGA1 is a mitochondrial 4-hydroxy-2-oxoglutarate aldolase catalyzing the fourth step in the hydroxyproline pathway. We investigated hydroxyproline metabolites in the urine of patients with primary hyperoxaluria type III using gas chromatography–mass spectroscopy. Significant increases in concentrations of 4-hydroxy-2-oxoglutarate and its precursor and derivative 4-hydroxyglutamate and 2,4-dihydroxyglutarate, respectively, were found in all patients as compared to carriers of the corresponding mutations or healthy controls. Despite a functional block in the conversion of hydroxyproline to glyoxylate—the immediate precursor of oxalate—the production of oxalate increases. To explain this apparent contradiction, we propose a model of glyoxylate compartmentalization in which cellular glyoxylate is normally prevented from contact with the cytosol where it can be oxidized to oxalate. We propose that HOGA1 deficiency results in the accumulation of 4-hydroxy-2-oxoglutarate in the mitochondria and its transport into the cytosol where it is converted to glyoxylate by a different cytosolic aldolase. In human hepatocyte cell lines, we detected a cytosolic 4-hydroxy-2-oxoglutarate aldolase activity not due to HOGA1. These studies provide a diagnostic tool for primary hyperoxaluria type III and shed light on glyoxylate metabolism and the pathogenesis of primary hyperoxalurias.






Similar content being viewed by others
References
Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D et al (2010) Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87:392–399
Monico CG, Rossetti S, Belostotsky R, Cogal AG, Herges RM, Seide BM, Olson JB, Bergstrahl EJ, Williams HJ, Haley WE et al (2011) Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin J Am Soc Nephrol 6:2289–2295
Riedel TJ, Johnson LC, Knight J, Hantgan RR, Holmes RP, Lowther WT (2011) Structural and biochemical studies of human 4-hydroxy-2-oxoglutarate aldolase: implications for hydroxyproline metabolism in primary hyperoxaluria. PLoS One 6:e26021
Williams EL, Bockenhauer D, Van't Hoff WG, Johri N, Laing C, Sinha MD, Unwin R, Viljoen A, Rumsby G (2012) The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficient in primary hyperoxaluria type 3. Nephrol Dial Transplant (in press)
Baker PR, Cramer SD, Kennedy M, Assimos DG, Holmes RP (2004) Glycolate and glyoxylate metabolism in HepG2 cells. Am J Physiol Cell Physiol 287:C1359–C1365
Coulter-Mackie MB (2006) 4-Hydroxyproline metabolism and glyoxylate production: a target for substrate depletion in primary hyperoxaluria? Kidney Int 70:1891–1893
Dijcker JC, Plantinga EA, van Baal J, Hendriks WH (2011) Influence of nutrition on feline calcium oxalate urolithiasis with emphasis on endogenous oxalate synthesis. Nutr Res Rev 22:1–15
Mdluli K, Booth MP, Brady RL, Rumsby G (2005) A preliminary account of the properties of recombinant human glyoxylate reductase (GRHPR), LDHA and LDHB with glyoxylate, and their potential roles in its metabolism. Biochim Biophys Acta 1753:209–216
Knight J, Jiang J, Assimos DG, Holmes RP (2006) Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney Int 70:1929–1934
Behnam JT, Williams EL, Brink S, Rumsby G, Danpure CJ (2006) Reconstruction of human hepatocyte glyoxylate metabolic pathways in stably transformed Chinese-hamster ovary cells. Biochem J 394:409–416
Yoshida Y, Holloway GP, Ljubicic V, Hatta H, Spriet LL, Hood DA, Bonen A (2007) Negligible direct lactate oxidation in subsarcolemmal and intermyofibrillar mitochondria obtained from red and white rat skeletal muscle. J Physiol 582:1317–1335
Holmes RS, Goldberg E (2009) Computational analyses of mammalian lactate dehydrogenases human, mouse, opossum and platypus LDHs. Comput Biol Chem 33:379–385
Samland AK, Sprenger GA (2006) Microbial aldolases as C–C bonding enzymes unknown treasures and new developments. Appl Microbiol Biotechnol 71:253–264
Machajewski TD, Wong CH (2000) The catalytic asymmetric aldol reaction. Angew Chem Int Ed Engl 39:1352–1375
Anderson M, Scholtz JM, Schuster SM (1985) Rat liver 4-hydroxy-2-ketoglutarate aldolase: purification and kinetic characterization. Arch Biochem Biophys 236:82–97
Kobes RD, Dekker EE (1969) 2-Keto-4-hydroxyglutarate aldolase of bovine liver. Purification, criteria of purity, and general properties. J Biol Chem 244:1919–1925
McLean A, Marwick MJ, Clayton BE (1973) Enzymes involved in phenylalanine metabolism in the human foetus and child. J Clin Pathol 26:678–683
Alaux S, Kusk M, Sagot E, Bolte J, Jensen AA, Bräuner-Osborne H, Gefflaut T, Bunch L (2005) Chemoenzymatic synthesis of a series of 4-substituted glutamate analogues and pharmacological characterization at human glutamate transporters subtypes 1-3. J Med Chem 48:7980–7992
Maitra U, Dekker EE (1963) Enzymatic steps in the conversion of gamma-hydroxyglutamate to glyoxylate and alanine. J Biol Chem 238:3660–3669
Birdsey GM, Lewin J, Holbrook JD, Simpson VR, Cunningham AA, Danpure CJ (2005) A comparative analysis of the evolutionary relationship between diet and enzyme targeting in bats, marsupials and other mammals. Proc Biol Sci 272:833–840
Danpure CJ, Fryer P, Jennings PR, Allsop J, Griffiths S, Cunningham A (1994) Evolution of alanine:glyoxylate aminotransferase 1 peroxisomal and mitochondrial targeting. A survey of its subcellular distribution in the livers of various representatives of the classes Mammalia, Aves and Amphibia. Eur J Cell Biol 64:295–313
Danpure CJ (1993) Primary hyperoxaluria type 1 and peroxisome-to-mitochondrion mistargeting of alanine:glyoxylate aminotransferase. Biochimie 75:309–315
Coulter-Mackie MB, Lian Q, Wong SG (2005) Overexpression of human alanine:glyoxylate aminotransferase in Escherichia coli: renaturation from guanidine-HCl and affinity for pyridoxal phosphate co-factor. Protein Expr Purif 41:18–26
Hopper ED, Pittman AM, Fitzgerald MC, Tucker CL (2008) In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J Biol Chem 283:30493–30502
Caldwell EF, Mayor LR, Thomas MG, Danpure CJ (2004) Diet and the frequency of the alanine:glyoxylate aminotransferase Pro11Leu polymorphism in different human populations. Hum Genet 115:504–509
Knight J, Easter LH, Neiberg R, Assimos DG, Holmes RP (2009) Increased protein intake on controlled oxalate diets does not increase urinary oxalate excretion. Urol Res 37:63–68
Acknowledgments
The authors are indebted to Prof. Hanna Mandel for helpful advice. Sections of this work were supported by the Victorian Government's Operational Infrastructure Support Program.
Conflict of interest
The authors declare that no conflict of interest exists.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 58 kb)
Rights and permissions
About this article
Cite this article
Belostotsky, R., Pitt, J.J. & Frishberg, Y. Primary hyperoxaluria type III—a model for studying perturbations in glyoxylate metabolism. J Mol Med 90, 1497–1504 (2012). https://doi.org/10.1007/s00109-012-0930-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0930-z