Abstract
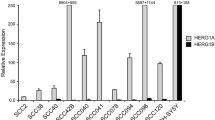
Compelling evidence indicates that the human ether-à-go-go voltage-gated potassium channels (hEAG1) may represent new valuable membrane therapeutic targets and diagnostic/prognostic biomarkers in various cancers. This study is the first to investigate the expression of hEAG1 potassium channel subunit in both primary tumors and HNSCC-derived cell lines to ascertain its clinical and biological role in tumor progression. Our findings demonstrate that hEAG1 is frequently aberrantly expressed in a high percentage of primary tumors (83 %, 45/54 cases) and HNSCC-derived cell lines (83 %, 10/12 cell lines). hEAG1 expression increased during HNSCC progression and was more frequent in advanced tumors. Strikingly, hEAG1 expression was also detected in a notable proportion (39 %, 17/44 cases) of patient-matched normal adjacent mucosa, whereas no expression was detected in normal epithelia from non-oncologic patients without exposure to tobacco carcinogens. In an attempt to identify the underlying mechanisms of aberrant hEAG1 expression in HNSCC, we found that hEAG1 gene copy gain occurred at a low frequency (15 %, 13/88 cases) in primary tumors but was not observed in early stages of HNSCC tumorigenesis. Furthermore, this study provides original evidence supporting the involvement of histone acetylation (i.e., H3Ac and H4K16Ac activating marks) in the regulation of hEAG1 expression in HNSCC. In addition, functional studies in HNSCC cells further revealed that hEAG1 expression is a biologically relevant feature that promotes cell proliferation and invasion, although independently of its ion-conducting function. Our findings strongly support the notion that hEAG1 may represent a promising candidate as tumor marker and membrane therapeutic target for HNSCC treatment.




Similar content being viewed by others
References
Forastiere A, Koch W, Trotti A, Sidransky D (2001) Head-and-neck cancer. N Engl J Med 345:1890–1900
Haddad RI, Shin DM (2008) Recent advances in head and neck cancer. N Engl J Med 359:1143–1154
Leemans CR, Braakhuis BJ, Brakenhoff RH (2011) The molecular biology of head and neck cancer. Nat Rev Cancer 11:9–22
Niemeyer BA, Mery L, Zawar C, Suckow A, Monje F, Pardo LA, Stuhmer W, Flockerzi V, Hoth M (2001) Ion channels in health and disease. EMBO Rep 2:568–573
O’Grady SM, Lee SY (2005) Molecular diversity and function of voltage-gated (Kv) potassium channels in epithelial cells. Int J Biochem Cell Biol 37:1578–1594
Kaczmarek LK (2006) Non-conducting functions of voltage-gated ion channels. Nat Rev Neurosci 7:761–771
Wang Z (2004) Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch 448:274–286
Kass RS (2005) The channelopathies: novel insights into molecular and genetic mechanisms of human disease. J Clin Invest 115:1986–1989
Wulff H, Castle NA, Pardo LA (2009) Voltage-gated potassium channels as therapeutic targets. Nat Rev Drug Discov 8:982–1001
Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W (2005) Role of voltage-gated potassium channels in cancer. J Membr Biol 205:115–124
Nilius B, Wohlrab W (1992) Potassium channels and regulation of proliferation of human melanoma cells. J Physiol 445:537–548
Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M et al (1998) herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res 58:815–822
Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC (2002) Functional upregulation of HERG K + channels in neoplastic hematopoietic cells. J Biol Chem 277:18528–18534
Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W (1999) Oncogenic potential of EAG K+ channels. EMBO J 18:5540–5547
Asher V, Sowter H, Shaw R, Bali A, Khan R (2010) Eag and HERG potassium channels as novel therapeutic targets in cancer. World J Surg Oncol 8:113
Pardo LA, Stühmer W (2008) Eag1: an emerging oncological target. Cancer Res 68:1611–1613
Hemmerlein B, Weseloh RM, Mello de Queiroz F, Knötgen H, Sánchez A, Rubio ME, Martin S, Schliephacke T, Jenke M, Heinz-Joachim-Radzun et al (2006) Overexpression of Eag1 potassium channels in clinical tumours. Mol Cancer 5:41
Mello de Queiroz F, Suarez-Kurtz G, Stühmer W, Pardo LA (2006) Ether à go-go potassium channel expression in soft tissue sarcoma patients. Mol Cancer 5:42
Agarwal JR, Griesinger F, Stühmer W, Pardo LA (2010) The potassium channel Ether à go-go is a novel prognostic factor with functional relevance in acute myeloid leukemia. Mol Cancer 9:18
Pedrero JM, Carracedo DG, Pinto CM, Zapatero AH, Rodrigo JP, Nieto CS, Gonzalez MV (2005) Frequent genetic and biochemical alterations of the PI 3-K/AKT/PTEN pathway in head and neck squamous cell carcinoma. Int J Cancer 114:242–248
Rodrigo JP, García-Carracedo D, García LA, Menéndez S, Allonca E, González MV, Fresno MF, Suárez C, García-Pedrero JM (2009) Distinctive clinicopathological associations of amplification of the cortactin gene at 11q13 in head and neck squamous cell carcinomas. J Pathol 217:516–523
Lansford CD, Grénman R, Bier H, Somers KD, Kim SY, Whiteside TL, Clayman GL, Welkoborsky HJ, Carey TE (1999) Head and neck cancers. In: Masters J, Palsson B (eds) Human cell culture. Kluwer, Dordrecht, pp 185–255
Hermsen MA, Joenje H, Arwert F, Welters MJ, Braakhuis BJ, Bagnay M, Westerveld A, Slater R (1996) Centromeric breakage as a major cause of cytogenetic abnormalities in oral squamous cell carcinoma. Gene Chromosome Cancer 15:1–9
Calvanese V, Lara E, Suárez-Alvarez B, Abu Dawud R, Vázquez-Chantada M, Martínez-Chantar ML, Embade N, López-Nieva P, Horrillo A, Hmadcha A et al (2010) Sirtuin 1 regulation of developmental genes during differentiation of stem cells. Proc Natl Acad Sci U S A 107:13736–13741
Gómez-Varela D, Zwick-Wallasch E, Knötgen H, Sánchez A, Hettmann T, Ossipov D, Weseloh R, Contreras-Jurado C, Rothe M, Stühmer W et al (2007) Monoclonal antibody blockade of the human Eag1 potassium channel function exerts antitumor activity. Cancer Res 67:7343–7349
Vindelov LL, Christensen IJ, Nissen NI (1983) Standardization of high-resolution flow cytometric DNA analysis by the simultaneous use of chicken and trout red blood cells as internal reference standards. Cytometry 3:328–331
Li LC, Dahiya R (2002) MethPrimer: designing primers for methylation PCRs. Bioinformatics 18:1427–1431
Weber C, Mello de Queiroz F, Downie BR, Suckow A, Stühmer W, Pardo LA (2006) Silencing the activity and proliferative properties of the human EagI potassium channel by RNA interference. J Biol Chem 281:13030–13037
Downie BR, Sánchez A, Knötgen H, Contreras-Jurado C, Gymnopoulos M, Weber C, Stühmer W, Pardo LA (2008) Eag1 expression interferes with hypoxia homeostasis and induces angiogenesis in tumors. J Biol Chem 283:36234–36240
Ousingsawat J, Spitzner M, Puntheeranurak S, Terracciano L, Tornillo L, Bubendorf L, Kunzelmann K, Schreiber R (2007) Expression of voltage-gated potassium channels in human and mouse colonic carcinoma. Clin Cancer Res 13:824–831
Restrepo-Angulo I, Sánchez-Torres C, Camacho J (2011) Human EAG1 potassium channels in the epithelial-to-mesenchymal transition in lung cancer cells. Anticancer Res 31:1265–1270
Namiki T, Yanagawa S, Izumo T, Ishikawa M, Tachibana M, Kawakami Y, Yokozeki H, Nishioka K, Kaneko Y (2005) Genomic alterations in primary cutaneous melanomas detected by metaphase comparative genomic hybridization with laser capture or manual microdissection: 6p gains may predict poor outcome. Cancer Genet Cytogenet 157:1–11
Bergamo NA, Rogatto SR, Poli-Frederico RC, Reis PP, Kowalski LP, Zielenska M, Squire JA (2000) Comparative genomic hybridization analysis detects frequent over-representation of DNA sequences at 3q, 7p, and 8q in head and neck carcinomas. Cancer Genet Cytogenet 119:48–55
Wagner JM, Hackanson B, Lübbert M, Jung M (2010) Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin Epigenetics 1:117–136
Lin H, Li Z, Chen C, Luo X, Xiao J, Dong D, Lu Y, Yang B, Wang Z (2011) Transcriptional and post-transcriptional mechanisms for oncogenic overexpression of ether à go-go K+ channel. PLoS One 6:e20362
Acknowledgements
We are grateful to all members of the Cancer Epigenetic Unit (IUOPA, Oviedo, Spain) and the Department of Molecular Biology of Neuronal Signals (Max-Planck-Institute, Göttingen, Germany), and Dr. Marta Alonso, Dr. Angel M. Nistal, Dr. José Luís Martínez and Ana Salas (SCT Facilities, University of Oviedo) for excellent technical assistance. We also thank Pablo Martínez-Camblor (OIB-CAIBER) for his assistance with statistical analyses, and Teresa Ortega and OIB staff for their administrative support. This work was supported by grants from Fondo de Investigación Sanitaria CP07/00032 and PI10/00157 (to J.M.G.P), PI11/00929 (to C.S.), ISCIII Fondos FEDER, RTICC (RD06/0020/0034) and Obra Social Cajastur-IUOPA. S.T.M. and S.A.T. are recipients of a fellowship from FICYT (BP08-007 and BP11-114).
Disclosure statement
L.A.P. is shareholder at iOnGen AG.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 576 kb)
Rights and permissions
About this article
Cite this article
Menéndez, S.T., Villaronga, M.Á., Rodrigo, J.P. et al. Frequent aberrant expression of the human ether à go-go (hEAG1) potassium channel in head and neck cancer: pathobiological mechanisms and clinical implications. J Mol Med 90, 1173–1184 (2012). https://doi.org/10.1007/s00109-012-0893-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-012-0893-0