Abstract
Current research, especially in oncology, increasingly focuses on the integration of quantitative, multiparametric and functional imaging data. In this fast-growing field of research, radiomics may allow for a more sophisticated analysis of imaging data, far beyond the qualitative evaluation of visible tissue changes. Through use of quantitative imaging data, more tailored and tumour-specific diagnostic work-up and individualized treatment concepts may be applied for oncologic patients in the future. This is of special importance in cross-sectional disciplines such as radiology and radiation oncology, with already high and still further increasing use of imaging data in daily clinical practice. Liver targets are generally treated with stereotactic body radiotherapy (SBRT), allowing for local dose escalation while preserving surrounding normal tissue. With the introduction of online target surveillance with implanted markers, 3D-ultrasound on conventional linacs and hybrid magnetic resonance imaging (MRI)-linear accelerators, individualized adaptive radiotherapy is heading towards realization. The use of big data such as radiomics and the integration of artificial intelligence techniques have the potential to further improve image-based treatment planning and structured follow-up, with outcome/toxicity prediction and immediate detection of (oligo)progression. The scope of current research in this innovative field is to identify and critically discuss possible application forms of radiomics, which is why this review tries to summarize current knowledge about interdisciplinary integration of radiomics in oncologic patients, with a focus on investigations of radiotherapy in patients with liver cancer or oligometastases including multiparametric, quantitative data into (radio)-oncologic workflow from disease diagnosis, treatment planning, delivery and patient follow-up.
Similar content being viewed by others
Introduction
With the introduction of radiomics, both oncologic radiology and radiation oncology have gained a highly promising tool for more sophisticated quantitative tumour analysis. Current research, especially in oncology, increasingly focuses on the integration of quantitative, multiparametric and functional imaging data. In this fast-growing field of research, radiomics may allow for an all-encompassing analysis of quantitative imaging data, far beyond the qualitative evaluation of visible tissue changes. Through use of multiparametric, quantitative imaging data, a more tailored and tumour-specific diagnostic work-up and individualized treatment concepts may be applied for oncologic patients in the future.
The scope of current research in this innovative field is to identify and critically discuss possible application forms of radiomics, which is why this review tries to summarize current knowledge about interdisciplinary integration of radiomics in oncologic patients. This review specifically focusses on investigations on radiotherapy in patients with liver cancer, including the (radio)-oncologic workflow from disease diagnosis, treatment planning, delivery and patient follow-up.
Radiomics includes not only the commonly known quantitative data features derived from the pixel grey-level histogram, i.e. mean, maximum, minimum and median parameters, but also the analysis of imaging data based on computerized mathematical and statistical feature extraction, describing further quantitative characteristics of the segmented regions with regard to tissue heterogeneity, compacity etc. [1]. Radiomic analysis of quantitative imaging parameters may further characterize both tumour and normal tissue and even predict tumour response and toxicity by incorporating these data into statistical or advanced machine learning models [2,3,4,5].
There are already some promising data about radiomics clarifying mammographic findings suspicious for cancer [6] or predicting mutational status in glioblastomas [7]. Additionally, there are still few but promising data regarding the use of radiomics in oncologic liver imaging [8]. Morphological and functional characterization of liver tumours with and without contrast-enhanced sequences is the state of the art in oncologic liver imaging. Recent radiomics studies demonstrated for the first time the predictive value for different liver tumours, such as the grade of hepatocellular carcinoma (HCC) or the differential diagnosis of other primary or secondary liver tumours and benign liver lesions [9,10,11,12,13]. Table 1 summarizes these studies.
The present review article aims at summarizing the work which has been done in the field of radiomics in liver imaging until today, with a special focus on relevant topics from the field of radiation therapy. Firstly, it will summarize the current indications for radiotherapy in the liver, before summarizing the current literature covering radiomics for treatment planning in the liver. A short section on radiomics for monitoring and follow-up will then be followed by a summary of radiomics in the imaging of the post-treatment liver. Finally, we will give a short overview about current limitations in the field of radiomics.
Indications for radiotherapy
With the introduction of image guidance and conformal radiotherapy techniques, the treatment of both primary and secondary liver tumours has experienced significant improvement over the past few years, leading to increased local control rates and decreased normal tissue toxicity [14,15,16,17,18,19].
Recent clinical data indicate that additional local therapy to each metastatic lesion can prolong the overall survival of oligometastatic patients [20,21,22]. Furthermore, immunotherapy enables new treatment options for several tumour entities, especially in combination with radiotherapy [23].
In patients with oligometastatic disease, especially hepatic metastases exhibit different treatment courses. Patients with up to five lesions are increasingly treated with aggressive metastasis-directed treatment options, improving survival in some patients, even in case of recurrent liver metastases [24, 25]. In comparison to other locally ablative treatment options, resection is anticipated for patients with isolated liver metastases, although being characterized with an increased post-procedure morbidity [24, 26].
Consequently, minimally invasive options like transarterial chemoembolization (TACE) and radiofrequency/microwave ablation (RFA/MWA) have been evaluated, demonstrating good high local control rates and safety records [27,28,29,30]. SBRT as a completely non-invasive procedure is an evolving alternative, showing similar or even better clinical outcomes [18, 31]. Unfortunately, to date, prospective data about the different local ablative treatment options are lacking. Nevertheless, ongoing technical improvements provide promising data, especially in the case of SBRT of liver metastases, with median overall survival rates of 31.5 months in colorectal cancer patients [32].
Primary liver cancers are generally intended to be resected. However, in case of inoperability, prospective studies on SBRT and SBRT with optional TACE in primary HCC showed promising 18-month and 3‑year overall survival rates of 72% and up to 67%, respectively [33, 34]. SBRT is even feasible in patients with advanced-stage HCC, with 3‑year overall survival (OS) rates of 24.3% and 3‑year local control rates of 78.1% [35]. Furthermore, there is increasing evidence that SBRT may even be superior to TACE regarding survival and recurrence, and especially after prior TAE/TACE treatment [36, 37]. In case of SBRT in cholangiocellular carcinoma (CCC) patients, 3‑year OS rates are about 21%, with increasing local control rates depending on delivered dose (biological effective radiation dose [max] >91 Gy [α/β = 10 Gy]) [38].
Although RFA is regarded as the main alternative treatment option in unresectable HCC, retrospective data indicate the possible superiority of SBRT as compared to RFA with regard to tumours >2 cm [18]. With competing data being published for the comparison of SBRT and RFA, prospective trials are needed [39]. If transplantation is indicated, a combination of neoadjuvant SBRT and TACE provides promising remission and reasonable overall survival rates [40, 41].
Radiomics for treatment planning
Target volume definition: automatic segmentation of target volumes and organs at risk
The functional capability of the liver to regenerate and proliferate has been used for a long time in liver surgery [42]. When it comes to a healthy liver, 80% of the organ can be removed. Although the whole liver exhibits a low radiation tolerance, potentially leading to the serious condition of radiotherapy-induced liver disease (RILD) [43,44,45,46,47,48,49], the regenerative potential and the parallel radiobiological character of the liver allows for application of high doses to a defined volume without compromising liver function [50].
Patients with liver metastases usually have a functionally “healthy” liver. Previous oncological treatments like chemotherapy or immunotherapy can influence liver function [51, 52]. How these previous therapies influence the individual radiation tolerance is still unknown and subject to research. In contrast to liver metastases, most patients with primary liver tumours have liver cirrhosis, which limits local ablative and surgical treatments due to the subsequently impaired liver function [53, 54]. Current understanding of the radiation-induced impairment of liver function sees hepatic veno-occlusive disease as the pathological hallmark of liver injury [55], while at the same time, the vulnerability of (hepato)biliary structures has to be taken into account, especially for centrally located liver cancer [56, 57].
Tolerance doses/dose constraints for therapy planning in organs at risk have been investigated for decades, which is why tolerance doses also rely on data being gained with different radiotherapy techniques and—most importantly—with irradiated volumes significantly larger than the volumes in modern radiotherapy techniques such as SBRT [58]. Consequently, organs at risk (OAR) constraints have to be re-evaluated in order to allow for more tailored treatment concepts and monitoring during treatment on the basis of clinical and multiparametric quantitative imaging data. With SBRT being characterized by different biological efficacy as compared to standard radiotherapy, dose escalation may be performed under consideration of dose constraints based on analyses on clinical toxicity [59,60,61,62,63].
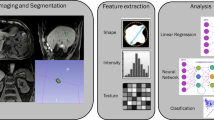
Radiomic analysis and its use during treatment planning might further contribute to improved dose escalation schemes and allow for future automation and increased robustness of target volume delineation. In addition, a more reliable identification of high-risk regions with possible tumour infiltration (clinical target volume [CTV]) might be enabled by radiomics, since radiomics extends beyond the visible tumour infiltration and provides quantitative data on potential tumour extension [64, 65]. An exemplified workflow for the extraction of radiomic features is shown in Fig. 1.
Exemplary radiomics workflow for liver imaging. Schematic illustration of the entire patient journey including image acquisition, analysis utilizing radiomics, and the derived patient-specific therapy and prognosis. Symptomatic patients undergo CT (computed tomography) or MR (magnetic resonance) scans. After image segmentation, radiomic features are extracted. High-level statistical modelling involving machine learning is applied for disease classification, patient clustering and individual risk stratification
Radiomic features and clinical parameters have been combined in a radiomics signature to preoperatively estimate early recurrence in patients with HCC [66, 67]. In addition, several promising studies (Table 2) have been conducted in liver tumours, where microscopic characteristics could be identified based on radiomics, thus potentially enabling the detection of microscopic tumour infiltration of healthy liver tissue with the inherent potential of more precise clinical target volume delineation in the future. Furthermore, evidence has been provided about prediction of microvascular invasion of HCC using radiomics on contrast-enhanced CT [68,69,70]. Using a radiomics approach based on contrast-enhanced T1-weighted MRI in the hepatobiliary phase, T‑cell infiltration in the tumour and peritumoural margin could be quantified [71]. Ex-vivo investigations in mice demonstrated the detection of microscopic tumour infiltration locations by the radiomic histogram feature skewness in liver single-photon emission CT (SPECT) imaging [72]. Thus, further specification of regions with a high risk of liver tumour infiltration may be enabled by radiomics in the future [70, 71].
Independently of current research on standardization of dose prescription, target volume definition is increasingly based on functional imaging, such as metabolic imaging with positron-emission tomography (PET) and functional sequences of MRI, including diffusion-weighted imaging (DWI) [73, 74]. PET-imaging allows for quantitative evaluation of metabolic function in the liver tumour and normal liver tissue, thus leading to the possibility of adapted target delineation and dose reduction in the normal liver tissue [75]. In contrast to PET-based image-guided treatment planning, contrast-enhanced MRI (including with liver-specific agents) is already part of daily clinical practice in treatment planning for patients with liver cancer [76].
Contrast-enhanced T1-weighted sequences allow for morphological target delineation, but functional imaging sequences such as DWI provide additional information about perfusion fractions by intravoxel incoherent motion (IVIM), cellularity by the ADC and even tissue complexity by kurtosis evaluation [77,78,79,80,81,82,83,84]. Consequently, research is conducted on incorporation of functional MRI sequences into the daily clinical practice of treatment planning of upper abdominal tumours [85]. Besides this, Liu et al. showed that synthetic CT datasets can be generated from MRI to ensure accurate liver SBRT [86].
Adding to radiomics, the increasingly used deep learning methods are able to learn directly from the data, thus circumventing the need for handcrafting of discriminative imaging features representing the key concept behind radiomics. Recently, automatic CTV segmentation has been shown by using convolutional neural networks (CNN), which appear to be especially useful for image segmentation tasks [87,88,89]. Deep learning-based auto-contouring of the tumour volume has been shown to be at least as efficient as manual contouring of the OAR for MRI-guided adaptive radiotherapy [90, 91]. Further promising performance for deep learning-based automatic segmentation approaches of the macroscopic gross tumour volume can be found in the ongoing “Liver Tumour Segmentation Challenge (LiTS)” (https://competitions.codalab.org/competitions/17094).
Independent of the images to be integrated into treatment planning, robust image registration (deformable or rigid registration) has to be performed to adjust for differences in image acquisition and organ movement, thus allowing for topographically correct target delineation [92].
Adaptive radiotherapy: dose painting
The idea of adaptive radiotherapy summarizes the goals of patient-specific and tumour-tailored treatment concepts, allowing for both adapted treatment planning with locally volitional dose escalation in malignancies and reduction of dose exposure in the surrounding normal tissue. This is already possible due to the highly conformal dose application in modern treatment techniques such as image-guided radiation therapy (IGRT) and SBRT. However, daily image guidance with cone beam computed tomography (CBCT) scans and consecutive plan adaptions request a high cost in resources while only providing low-resolution CBCT images. The latest introduction of hybrid MR-guided radiotherapy gadgets might finally allow for online imaging and plan adaption based on high-resolution images, especially with respect to the soft tissue of upper abdominal organs [93]. Furthermore, this also allows for taking into account tumour heterogeneity based on quantitative, functional imaging data, exceeding the purely morphological characteristics and potentially allowing for earlier evaluation of tumour response and local control failure already at the beginning of radiotherapy and in the meantime (Table 3; [91, 94]).
Independent of general outcome analysis, the incorporation of radiomics into treatment planning may further improve the analysis and prediction of normal tissue toxicity, as proposed by the QUANTEC group (Quantitative Analysis of Normal Tissue Effects in the Clinic) [95, 96]. There are already promising data with regard to toxicity after radiotherapy of head and neck and lung cancers [97, 98]. However, regarding toxicity after treatment of liver tumours, there is still little data available and future investigations are needed. Cai et al. demonstrated the prediction of liver failure after hepatectomy in patients with HCC by preoperative radiomics-based nomograms [99]. A predictive nomogram and a CNN including imaging data with high performance for toxicity prediction after liver SBRT are already available [57, 100]. Generally, toxicity analysis in healthy liver tissue should be improved even more, since MRI enables accurate liver function analysis and radiomics analysis allows for accurate staging of liver fibrosis and may prevent and, vice versa, predict RILD [101,102,103,104].
Monitoring/follow-up
First promising results regarding the predictive potential of radiomics for local response after radiotherapy and TACE have recently been demonstrated [105,106,107] and are listed in Table 4. Treatment response of liver metastases after TACE has been determined using a radiomics-based analysis resulting in area under the curve (AUC) in receiver operating characteristics (ROC) of up to 0.83 [105]. Radiomic features have been integrated into multivariate models predicting local control and overall survival rates after radiotherapy of HCC with an AUC of 0.80 [106]. Furthermore, by combining radiomics features with clinical data, survival prediction might even be improved in patients with HCC [13, 107, 108]. Even in case of cholangiocarcinoma, pre-operative MRI was able to predict early recurrence, especially in combination with immunohistochemical markers [109].
Imaging of the post-treatment liver
In addition to a temporary/reversible decline in metabolic function, liver tissue is characterized by distinct macroscopic, microscopic and CT/MR-morphological changes after radiotherapy; exemplary changes are given in Fig. 2. In patients with sufficient baseline liver function prior to radiotherapy, a compensatory hypertrophy of the untreated liver may occur after radiotherapy [110]. Repetitive imaging during and after radiotherapy demonstrated that changes in metabolic liver function are also accompanied by distinct changes in quantitative imaging data of the liver tumour and the normal liver tissue [53, 111,112,113,114].
Longitudinal changes of a hepatic metastasis in the right liver lobe after stereotactic radiotherapy (SBRT). MRI sequences: diffusion-weighted imaging (DWI) transverse (a–c), contrast-enhanced T1-weighted sequence (portal-venous phase) transverse (d–f) and coronal (g–i). MRI prior to SBRT (a, d, g), 3 months after SBRT (b, e, h) and 12 months after SBRT (c, f, i). Morphological response of DWI restriction, T1‑w hypointensity after SBRT with longitudinal reduction of peritumoral changes of the normal tissue. White arrows highlight the region of interest including the hepatic metastasis in the right liver lobe and the peritumoral changes after SBRT
Nevertheless, there are still few data about quantitative imaging encompassing the whole treatment course of liver tumours, especially with respect to normal tissue alterations. Multiparametric imaging might allow for both specification in staging examinations and acceleration by the use of quantitative imaging parameters, potentially replacing extensive amounts of qualitative imaging sequences for visible, mainly qualitative evaluation of the tumour and normal tissue. Sequences such as DWI with quantitative ADC maps are characterized by these possibilities and allow for functional analysis of the tumour response after SBRT [115].
As a consequence, integration of this quantitative data might lead to an improvement of oncologic patient management in future, especially with respect to the large amount of radiological data in image-guided radiation oncology. Investigations in this field emphasize the role of big data in oncology. With regard to radiation oncology, the so-called radiomics concept with computerized algorithm-based parameters may be successfully integrated into daily clinical practice, supporting decision-making and improving the workflow of radiation therapy.
Current limitations of radiomics
As reviewed above, radiomics for radiotherapy of liver tumours is highly promising, but we are still in need of further data about its validity and optimal usage for a reliable translation to daily clinical practice. The correct and robust application of radiomics analysis has to be investigated, since the algorithm-based analysis of quantitative date is not standardized within different institutions, or even within single institutions, and can easily be performed differently. Independently of software- and hardware-induced variability [116,117,118,119,120,121], texture analysis is also hindered by uncertainties in patient immobilization and organ movements, especially with regard to MRI examinations [122, 123]. On top of this, its usage for treatment decisions and treatment planning has to be investigated in prospective trials including ex-vivo, in-vivo volunteer and in-vivo patient examinations allowing for founded conclusions about its usage. Furthermore, the analysis of radiomics necessitates sufficient informational technique (IT) infrastructure with high data storage capacity and computational performance in image analysis, which is why the introduction of radiomics analysis in radiation oncology requests an IT structure similar to the one in radiology institutions.
Independent of providing sufficient software and hardware, incorporation of radiomics into radiation oncology also necessitates interdisciplinary teams, including medical doctors (clinical radiation oncologists, clinical radiologists), medical physicists and most importantly, computer scientists. The evaluation of radiomics on the basis of incorporating the imaging data together with clinical and histological data into artificial intelligence techniques, such as deep convolutional neural networks, is of utmost importance.
Conclusion
Due to the introduction of modern radiation treatment techniques such as SBRT and IGRT, radiotherapy is capable of successfully treating both primary and secondary liver tumours, with promising local control rates. With the possibilities of multiparametric, quantitative data, including the deeper radiomics analysis, information exceeding qualitative evaluation of visible changes may be included into oncologic radiology and radiation oncology. Hybrid MR-guided radiotherapy gadgets may summarize these techniques and, together with further evaluations with artificial intelligence, patient-specific and tumour-tailored radiation treatment may become a reality.
As a consequence, prospective multi-institutional trials for liver radiotherapy are needed, with standardized image acquisition integrating radiomics quality scores to improve the research quality and to increase the influence of radiomics, further analysing radiomics’ impact in patients with liver tumours and evaluating the true potential of the predictive models.
References
Lambin P et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48(4):441–446
Bickelhaupt S et al (2017) Prediction of malignancy by a radiomic signature from contrast agent-free diffusion MRI in suspicious breast lesions found on screening mammography. J Magn Reson Imaging 46(2):604–616
Rosenstein BS et al (2014) Radiogenomics: radiobiology enters the era of big data and team science. Int J Radiat Oncol Biol Phys 89(4):709–713
Cunliffe A et al (2015) Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys 91(5):1048–1056
Perrin T et al (2018) Short-term reproducibility of radiomic features in liver parenchyma and liver malignancies on contrast-enhanced CT imaging. Abdom Radiol 43(12):3271–3278
Bickelhaupt S et al (2018) Radiomics based on adapted diffusion Kurtosis imaging helps to clarify most mammographic findings suspicious for cancer. Radiology 287(3):761–770
Li ZC et al (2018) Multiregional radiomics profiling from multiparametric MRI: Identifying an imaging predictor of IDH1 mutation status in glioblastoma. Cancer Med 7(12):5999–6009
Andrea C‑G et al (2020) Developments in predictive biomarkers for hepatocellular carcinoma therapy. Expert Rev Anticancer Ther 20(1):63–74
Lewis S et al (2019) Volumetric quantitative histogram analysis using diffusion-weighted magnetic resonance imaging to differentiate HCC from other primary liver cancers. Abdom Radiol 44(3):912–922
Wu J et al (2019) Radiomics-based classification of hepatocellular carcinoma and hepatic haemangioma on precontrast magnetic resonance images. BMC Med Imaging 19(1):23
Oyama A et al (2019) Hepatic tumor classification using texture and topology analysis of non-contrast-enhanced three-dimensional T1-weighted MR images with a radiomics approach. Sci Rep 9(1):8764
Wu M et al (2019) Predicting the grade of hepatocellular carcinoma based on non-contrast-enhanced MRI radiomics signature. Eur Radiol 29(6):2802–2811
Guo D et al (2019) Radiomics analysis enables recurrence prediction for hepatocellular carcinoma after liver transplantation. Eur J Radiol 117:33–40
Gerum S et al (2018) Stereotactic body radiation therapy (SBRT) in patients with hepatocellular carcinoma and oligometastatic liver disease. Radiat Oncol 13(1):100
Mahadevan A et al (2018) Stereotactic Body Radiotherapy (SBRT) for liver metastasis—clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol 13(1):26
Nabavizadeh N et al (2018) Safety and efficacy of accelerated hypofractionation and stereotactic body radiation therapy for hepatocellular carcinoma patients with varying degrees of hepatic impairment. Int J Radiat Oncol Biol Phys 100(3):577–585
Andratschke N et al (2018) The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer 18(1):283
Wahl DR et al (2016) Outcomes after stereotactic body radiotherapy or radiofrequency ablation for hepatocellular carcinoma. J Clin Oncol 34(5):452–459
Klein J et al (2015) Prospective longitudinal assessment of quality of life for liver cancer patients treated with stereotactic body radiation therapy. Int J Radiat Oncol Biol Phys 93(1):16–25
Ost P et al (2018) Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: a prospective, randomized, multicenter phase II trial. J Clin Oncol 36(5):446–453
Palma DA et al (2019) Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet 393(10185):2051–2058
Gomez DR et al (2019) Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol 37(18):1558–1565
Deutsch E et al (2019) Optimising efficacy and reducing toxicity of anticancer radioimmunotherapy. Lancet Oncol 20(8):e452–e463
Dupre A et al (2017) Curative-intent treatment of recurrent colorectal liver metastases: a comparison between ablation and resection. Eur J Surg Oncol 43(10):1901–1907
Klement RJ et al (2019) The impact of local control on overall survival after stereotactic body radiotherapy for liver and lung metastases from colorectal cancer: a combined analysis of 388 patients with 500 metastases. BMC Cancer 19(1):173
Van Cutsem E et al (2006) Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer 42(14):2212–2221
Rusthoven KE et al (2009) Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 27(10):1572–1578
Riemsma RP et al (2013) Transarterial (chemo)embolisation versus no intervention or placebo intervention for liver metastases. Cochrane Database Syst Rev Cd009498:4
Cirocchi R et al (2012) Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev Cd006317:6
Levy J et al (2018) Intra-arterial therapies for unresectable and chemorefractory colorectal cancer liver metastases: a systematic review and meta-analysis. HPB 20(10):905–915
Franzese C et al (2018) Liver metastases from colorectal cancer: propensity score-based comparison of stereotactic body radiation therapy vs. microwave ablation. J Cancer Res Clin Oncol 144(9):1777–1783
Petrelli F et al (2018) Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother Oncol 129(3):427–434
Takeda A et al (2016) Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer 122(13):2041–2049
Durand-Labrunie J et al (2020) Curative irradiation treatment of hepatocellular carcinoma: a multicenter phase 2 trial. Int J Radiat Oncol Biol Phys 3016(19):34512–34512
Lo CH et al (2017) Survival and prognostic factors for patients with advanced hepatocellular carcinoma after stereotactic ablative radiotherapy. PLoS ONE 12(e0177793):5
Shen P‑C et al (2019) Comparison of stereotactic body radiation therapy and transarterial chemoembolization for unresectable medium-sized hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 105(2):307–318
Comito TLM, Franzese C, Clerici E, Pedicini V, Poretti D, Solbiati L, Rimassa L, Scorsetti M (2020) PB02-02 SBRT vs TAE/TACE in Hepatocellular carcinoma: results from a Phase III trial (NTC02323360). European Association for the Study of the Liver (EASL), Prague
Brunner TB et al (2019) Stereotactic body radiotherapy dose and its impact on local control and overall survival of patients for locally advanced intrahepatic and extrahepatic cholangiocarcinoma. Radiother Oncol 132:42–47
Rajyaguru DJ et al (2018) Radiofrequency ablation versus stereotactic body radiotherapy for localized hepatocellular carcinoma in nonsurgically managed patients: analysis of the national cancer database. J Clin Oncol 36(6):600–608
Honda Y et al (2013) Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol 28(3):530–536
Jacob R et al (2015) Adjuvant stereotactic body radiotherapy following transarterial chemoembolization in patients with non-resectable hepatocellular carcinoma tumours of 〉/= 3 cm. HPB 17(2):140–149
Michalopoulos GK (2010) Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol 176(1):2–13
Dawson LA, Ten Haken RK (2005) Partial volume tolerance of the liver to radiation. Semin Radiat Oncol 15(4):279–283
Jung J et al (2013) Radiation-induced liver disease after stereotactic body radiotherapy for small hepatocellular carcinoma: clinical and dose-volumetric parameters. Radiat Oncol 8:249
Cheng JC et al (2002) Radiation-induced liver disease after three-dimensional conformal radiotherapy for patients with hepatocellular carcinoma: dosimetric analysis and implication. Int J Radiat Oncol Biol Phys 54(1):156–162
Su TS, Luo R, Liang P, Cheng T, Zhou Y, Huang Y (2018) A prospective cohort study of hepatic toxicity after stereotactic body radiation therapy for hepatocellular carcinoma. Radiother Oncol 129(1):136–142. https://doi.org/10.1016/j.radonc.2018.02.031
Gkika E et al (2018) The role of albumin-bilirubin grade and inflammation-based index in patients with hepatocellular carcinoma treated with stereotactic body radiotherapy. Strahlenther Onkol 194(5):403–413
Ito K et al (2019) Whole-liver radiotherapy for diffuse liver metastases improves liver enzymes and related factors. Acta Oncol 58(4):512–514
Miften M, Vinogradskiy Y, Moiseenko V, et al (2018) Radiation Dose-Volume Effects for Liver SBRT [published online ahead of print, 2018 Jan 6]. Int J Radiat Oncol Biol Phys S0360–3016(17)34527–34523. https://doi.org/10.1016/j.ijrobp.2017.12.290
McPartlin A et al (2017) Long-term outcomes of phase 1 and 2 studies of SBRT for hepatic colorectal metastases. Int J Radiat Oncol Biol Phys 99(2):388–395
Hiwatashi K et al (2016) The evaluation of liver function and surgical influence by ICGR15 after chemotherapy for colorectal liver metastases. J Cancer 7(5):595–599
Huffman BM et al (2018) Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma: natural progression and management. Am J Clin Oncol 41(8):760–765
Dreher C et al (2016) Metabolic liver function after stereotactic body radiation therapy for hepatocellular carcinoma. Acta Oncol 55(7):886–891
Toesca DAS et al (2017) Assessment of hepatic function decline after stereotactic body radiation therapy for primary liver cancer. Pract Radiat Oncol 7(3):173–182
DeLeve LD, Shulman HM, McDonald GB (2002) Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 22(1):27–42
Osmundson EC et al (2015) Predictors of toxicity associated with stereotactic body radiation therapy to the central hepatobiliary tract. Int J Radiat Oncol Biol Phys 91(5):986–994
Toesca DA et al (2017) Central liver toxicity after SBRT: an expanded analysis and predictive nomogram. Radiother Oncol 122(1):130–136
Koay EJ, Owen D, Das P (2018) Radiation-induced liver disease and modern radiotherapy. Semin Radiat Oncol 28(4):321–331
Hanna GG et al (2018) UK consensus on normal tissue dose constraints for stereotactic radiotherapy. Clin Oncol 30(1):5–14
Pan CC et al (2010) Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 76(3 Suppl):S94–100
Grimm J et al (2011) Dose tolerance limits and dose volume histogram evaluation for stereotactic body radiotherapy. J Appl Clin Med Phys 12(2):3368
Méndez Romero A, de Man RA (2016) Stereotactic body radiation therapy for primary and metastatic liver tumors: from technological evolution to improved patient care. Best Pract Res Clin Gastroenterol 30(4):603–616
Asbell SO et al (2016) Introduction and clinical overview of the DVH risk map. Semin Radiat Oncol 26(2):89–96
Ma S, Xie H, Wang H, et al (2019) MRI-Based Radiomics Signature for the Preoperative Prediction of Extracapsular Extension of Prostate Cancer. J Magn Reson Imaging 50(6):1914–1925. https://doi.org/10.1002/jmri.26777
Li M et al (2016) Computed tomography texture analysis to facilitate therapeutic decision making in hepatocellular carcinoma. Oncotarget 7(11):13248–13259
Shan Q‑Y et al (2019) CT-based peritumoral radiomics signatures to predict early recurrence in hepatocellular carcinoma after curative tumor resection or ablation. Cancer Imaging 19(1):11–11
Zhou Y et al (2017) CT-based radiomics signature: a potential biomarker for preoperative prediction of early recurrence in hepatocellular carcinoma. Abdom Radiol 42(6):1695–1704
Xu X et al (2019) Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma. J Hepatol 70(6):1133–1144
Bakr S et al (2017) Noninvasive radiomics signature based on quantitative analysis of computed tomography images as a surrogate for microvascular invasion in hepatocellular carcinoma: a pilot study. J Med Imaging 4(4):41303–41303
Peng J et al (2018) A radiomics nomogram for preoperatively predicting prognosis of patients in hepatocellular carcinoma. Transl Cancer Res 7(4):936–946
Chen S et al (2019) Pretreatment prediction of immunoscore in hepatocellular cancer: a radiomics-based clinical model based on Gd-EOB-DTPA-enhanced MRI imaging. Eur Radiol 29(8):4177–4187
Veres DS et al (2019) Radiomic detection of microscopic tumorous lesions in small animal liver SPECT imaging. EJNMMI Res 9(1):67
Houweling AC et al (2013) FDG-PET and diffusion-weighted MRI in head-and-neck cancer patients: implications for dose painting. Radiother Oncol 106(2):250–254
Prezzi D et al (2018) The impact of MRI sequence on tumour staging and gross tumour volume delineation in squamous cell carcinoma of the anal canal. Eur Radiol 28(4):1512–1519
Fode MM et al (2017) A phase I study on stereotactic body radiotherapy of liver metastases based on functional treatment planning using positron emission tomography with 2‑[(18)F]fluoro-2-deoxy-d-galactose. Acta Oncol 56(11):1614–1620
Thian YL, Riddell AM, Koh DM (2013) Liver-specific agents for contrast-enhanced MRI: role in oncological imaging. Cancer Imaging 13(4):567–579
Joo I et al (2016) Monitoring vascular disrupting therapy in a rabbit liver tumor model: relationship between tumor perfusion parameters at IVIM diffusion-weighted MR imaging and those at dynamic contrast-enhanced MR imaging. Radiology 278(1):104–113
Woo S et al (2014) Intravoxel incoherent motion diffusion-weighted MR imaging of hepatocellular carcinoma: correlation with enhancement degree and histologic grade. Radiology 270(3):758–767
Bickelhaupt S et al (2018) Radiomics based on adapted diffusion Kurtosis imaging helps to clarify most mammographic findings suspicious for cancer. Radiology 287(3):761–770
Sun K et al (2015) Breast cancer: diffusion Kurtosis MR imaging—diagnostic accuracy and correlation with clinical-pathologic factors. Radiology 277(1):46–55
d’Assignies G et al (2013) High sensitivity of diffusion-weighted MR imaging for the detection of liver metastases from neuroendocrine tumors: comparison with T2-weighted and dynamic gadolinium-enhanced MR imaging. Radiology 268(2):390–399
Surov A, Meyer HJ, Wienke A (2017) Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget 8(35):59492–59499
Jensen JH et al (2005) Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med 53(6):1432–1440
Le Bihan D et al (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168(2):497–505
Dalah E et al (2014) Variability of target and normal structure delineation using multimodality imaging for radiation therapy of pancreatic cancer. Int J Radiat Oncol Biol Phys 89(3):633–640
Liu Y et al (2019) MRI-based treatment planning for liver stereotactic body radiotherapy: validation of a deep learning-based synthetic CT generation method. BJR 92(1100):20190067–20190067
Deng Z, Guo Q, Zhu Z (2019) Dynamic regulation of level set parameters using 3D Convolutional neural network for liver tumor segmentation. J Healthc Eng p:4321645
Vivanti R et al (2018) Patient-specific and global convolutional neural networks for robust automatic liver tumor delineation in follow-up CT studies. Med Biol Eng Comput 56(9):1699–1713
Vorontsov E et al (2017) Metastatic liver tumour segmentation with a neural network-guided 3D deformable model. Med Biol Eng Comput 55(1):127–139
Fu Y et al (2018) A novel MRI segmentation method using CNN-based correction network for MRI-guided adaptive radiotherapy. Med Phys 45(11):5129–5137
Zhang Y et al (2018) A knowledge-based approach to automated planning for hepatocellular carcinoma. J Appl Clin Med Phys 19(1):50–59
Velec M et al (2017) Validation of biomechanical deformable image registration in the abdomen, thorax, and pelvis in a commercial radiotherapy treatment planning system. Med Phys 44(7):3407–3417
Witt JS, Rosenberg SA, Bassetti MF (2020) MRI-guided adaptive radiotherapy for liver tumours: visualising the future. Lancet Oncol 21(2):e74–e82
Dogan N et al (2019) EP-2023 Predictive value of delta-radiomics features extracted from MR Images in image-guided liver SBRT. Radiother Oncol 133:S1109–S1110
Bentzen SM et al (2010) Quantitative analyses of normal tissue effects in the clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys 76(3 Suppl):S3–S9
El Naqa I et al (2017) Radiogenomics and radiotherapy response modeling. Phys Med Biol 62(16):R179–R206
Cunliffe A et al (2015) Lung texture in serial thoracic computed tomography scans: correlation of radiomics-based features with radiation therapy dose and radiation pneumonitis development. Int J Radiat Oncol Biol Phys 91(5):1048–1056
Scalco E et al (2013) Texture analysis for the assessment of structural changes in parotid glands induced by radiotherapy. Radiother Oncol 109(3):384–387
Cai W et al (2019) A radiomics-based nomogram for the preoperative prediction of posthepatectomy liver failure in patients with hepatocellular carcinoma. Surg Oncol 28:78–85
Ibragimov B et al (2018) Development of deep neural network for individualized hepatobiliary toxicity prediction after liver SBRT. Med Phys 45(10):4763–4774
Park HJ et al (2019) Radiomics analysis of gadoxetic acid-enhanced MRI for staging liver fibrosis. Radiology 290(2):380–387
Ding Y et al (2015) Assessing liver function in patients with HBV-related HCC: a comparison of T(1) mapping on Gd-EOB-DTPA-enhanced MR imaging with DWI. Eur Radiol 25(5):1392–1398
Katsube T et al (2011) Estimation of liver function using T1 mapping on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Invest Radiol 46(4):277–283
Toesca DAS et al (2018) Strategies for prediction and mitigation of radiation-induced liver toxicity. J Radiat Res 59(suppl_1):i40–i49
Reimer RP, Reimer P, Mahnken AH (2018) Assessment of therapy response to transarterial radioembolization for liver metastases by means of post-treatment MRI-based texture analysis. Cardiovasc Intervent Radiol 41(10):1545–1556
Cozzi L et al (2017) Radiomics based analysis to predict local control and survival in hepatocellular carcinoma patients treated with volumetric modulated arc therapy. BMC Cancer 17(1):829
Kim J et al (2018) Predicting survival using pretreatment CT for patients with hepatocellular carcinoma treated with transarterial chemoembolization: comparison of models using radiomics. Ajr Am J Roentgenol 211(5):1026–1034
Mokrane F‑Z et al (2020) Radiomics machine-learning signature for diagnosis of hepatocellular carcinoma in cirrhotic patients with indeterminate liver nodules. Eur Radiol 30(1):558–570
Zhao L et al (2019) Prediction for early recurrence of intrahepatic mass-forming cholangiocarcinoma: quantitative magnetic resonance imaging combined with prognostic immunohistochemical markers. Cancer Imaging 19(1):49–49
Ohara K et al (1997) Radiation tolerance of cirrhotic livers in relation to the preserved functional capacity: analysis of patients with hepatocellular carcinoma treated by focused proton beam radiotherapy. Int J Radiat Oncol Biol Phys 38(2):367–372
Mastrocostas K et al (2019) Imaging post-stereotactic body radiation therapy responses for hepatocellular carcinoma: typical imaging patterns and pitfalls. Abdom Radiol 44(5):1795–1807
Boda-Heggemann J et al (2016) MRI morphologic alterations after liver SBRT: direct dose correlation with intermodal matching. Strahlenther Onkol 192(9):641–648
Boda-Heggemann J et al (2018) Direct dose correlation of MRI morphologic alterations of healthy liver tissue after robotic liver SBRT. Strahlenther Onkol 194(5):414–424
Sanuki N et al (2014) Threshold doses for focal liver reaction after stereotactic ablative body radiation therapy for small hepatocellular carcinoma depend on liver function: evaluation on magnetic resonance imaging with Gd-EOB-DTPA. Int J Radiat Oncol Biol Phys 88(2):306–311
Sampath S, Rahmanuddin S, Sahoo P, et al (2019) Change in Apparent Diffusion Coefficient Is Associated With Local Failure After Stereotactic Body Radiation Therapy for Non-Small Cell Lung Cancer: A Prospective Clinical Trial. Int J Radiat Oncol Biol Phys 105(3):659–663. https://doi.org/10.1016/j.ijrobp.2019.06.2536
Zwanenburg A, Leger S, Vallières M, Löck S (2016) Image biomarker standardisation initiative. (arXiv:1612.07003 [cs.CV])
Zhovannik I et al (2019) Learning from scanners: Bias reduction and feature correction in radiomics. Clinical and Translational Radiation Oncology 19:33–38
Zhao B et al (2016) Reproducibility of radiomics for deciphering tumor phenotype with imaging. Sci Rep 6:23428–23428
Zhao B et al (2014) Exploring variability in CT characterization of tumors: a preliminary phantom study. Transl Oncol 7(1):88–93
Traverso A et al (2018) Repeatability and Reproducibility of Radiomic Features: A Systematic Review. Int J Radiat Oncol Biol Phys 102(4):1143–1158
Shafiq-Ul-Hassan M et al (2017) Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys 44(3):1050–1062
Baessler B, Weiss K, Pinto Dos DS (2019) Robustness and reproducibility of radiomics in magnetic resonance imaging: a phantom study. Invest Radiol 54(4):221–228
Um H et al (2019) Impact of image preprocessing on the scanner dependence of multi-parametric MRI radiomic features and covariate shift in multi-institutional glioblastoma datasets. Phys Med Biol 64(16):165011–165011
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors were involved in writing and revising the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
C. Dreher, P. Linde, J. Boda-Heggemann and B. Baessler declare that they have no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dreher, C., Linde, P., Boda-Heggemann, J. et al. Radiomics for liver tumours. Strahlenther Onkol 196, 888–899 (2020). https://doi.org/10.1007/s00066-020-01615-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00066-020-01615-x