Abstract
Multiorgan failure is among the most frequent reasons of death in critically ill patients. Based on extensive and long-term use of renal replacement therapy, extracorporeal organ support became available for other organ failures. Initially, most of these techniques (e.g. extracorporeal membrane oxygenation, extracorporeal CO2 removal [ECCO2R] and extracorporeal liver support) were used as stand-alone single organ support systems. Considering multiple interactions between native organs (“crosstalk”), combined or integrated extracorporeal organ support (ECOS) devices are intriguing. The concept of multiple organ support therapy (MOST) providing simultaneous and combined support for different failing organs was described more than 15 years ago by Ronco and Bellomo. This concept also implicates overcoming the “compartmentalized” approach provided by different single organ specialized professionals by a multidisciplinary and multiprofessional strategy. The idea of MOST is supported by the failure of several recent studies on single organ support including liver and lung support. Improvement of outcome by ECOS necessarily depends on optimized patient selection, integrated organ support and limitation of its side effects. This implicates challenges for engineers, industry and healthcare professionals. From a technical viewpoint, modular combination of pre-existing technologies such as renal replacement, albumin-dialysis, ECCO2R and potentially cytokine elimination can be considered as a first step. While this allows for stepwise and individual combination of standard organ support facilities, it carries the disadvantage of large extracorporeal blood volume and surfaces as well as additive costs. The more intriguing next step is an integrated platform providing the capacity of multiple organ support within one device. (This article is freely available.)
Zusammenfassung
Das Multiorganversagen ist eine der häufigsten Todesursachen auf der Intensivstation. Die breite Anwendung der Nierenersatztherapie bei akutem und chronischem Nierenversagen ebnete den Weg für andere extrakorporale Organersatzverfahren. Diese wurden zunächst überwiegend als Einzelorganersatztherapien eingesetzt (extrakorporale Membranoxygenierung, extrakorporale CO2-Entfernung [ECCO2R] sowie extrakorporaler Leberersatz). Im Hinblick auf multiple Interaktionen zwischen den Organsystemen („crosstalk“) sind kombinierte bzw. integrierte Organersatzverfahren von großem Interesse. Das Konzept der „multiple organ support therapy“ (MOST) mit kombiniertem Organersatz wurde vor über 15 Jahren von Ronco und Bellomo erstbeschrieben. Dieses Konzept ersetzt den Ansatz der „Kompartimentalisierung“ mit Ersatz einzelner Organversagen im Rahmen der jeweiligen speziellen Verfahren durch eine multidisziplinäre, multiprofessionelle Vorgehensweise. Die Strategie der MOST gewann nach dem Scheitern mehrerer jüngster Studien zum Einzelorganersatz (v. a. Leber- bzw. Lungenersatz) zunehmend an Bedeutung. Der zukünftige Erfolg dieses Konzepts des integrierten Organersatzes hängt dabei auch von einer optimierten Patientenauswahl und einer Limitierung von Nebenwirkungen des Verfahrens ab. Dies bringt zwangsläufig Herausforderungen für Ingenieure, Industrie und Heilberufe mit sich. Technisch ist eine bloße Kombination von vorbestehenden Verfahren wie Nieren- oder Leberersatz, CO2-Entfernung und ggf. Zytokinelimination nur ein erster Schritt. Auch wenn dies eine schrittweise und individualisierte Kombination von vorhandenen Organunterstützungstherapien bedeutet, ergibt sich daraus der Nachteil eines hohen extrakorporalen Blutvolumens, großer künstlicher Oberflächen und additiver Kosten für die einzelnen Verfahren. Der notwendige nächste Schritt sind integrierte Verfahren, die einen Multiorganersatz in einem Gerät ermöglichen.
Similar content being viewed by others
Introduction
Synchronous or sequential failure of different organs has been termed multiorgan dysfunction syndrome (MODS) or multiorgan failure (MOF). It was first described 50 years ago as a syndrome with “respiratory failure, hypotension, sepsis and jaundice” [1]. MOF is the most frequent cause of mortality in critically ill patients [2]. An increasing number of extracorporeal organ support modalities is intriguing to provide extracorporeal organ support (ECOS) [2,3,4,5,6]. This review reports on recent advances in diagnosis and therapy of MOF.
History of extracorporeal organ support
In the last two decades, experimental research as well as clinical data (e.g. the SOFA database) emphasized that organ failure is rarely a “stand-alone” organ failure [7]. By contrast, combined and interacting organ failures are frequent. While humoral and cellular interaction—termed “organ crosstalk”—has been characterized more recently [3], syndromic combined organ failure has been described for a long time. For example, hepatorenal syndrome is associated with a dramatic decrease of survival compared to single organ failure of a compensated cirrhosis.
Even if the term extracorporeal organ support has been recently generalized [5], this concept was introduced about 100 years ago, when the first devices for renal replacement therapy (RRT) were investigated. Based on the theories from Graham, and the experiences from Haas and Abel, Rowntree and Turner, RRT became widely available starting in the 1950s and part of clinical routine thanks to the designs from Kollf [8]. Continuous technological improvements permitted the application of intermittent modalities for chronic patients by Scribner in 1960, the treatment of fluid overload by ultrafiltration by Silverstein in 1974, employing what is now known as slow continuous ultrafiltration (SCUF), the first continuous renal replacement therapy (CRRT) by Kramer in 1977 and newer techniques as the slow extended daily dialysis (SLEDD) introduced by Depner and Golper in 1998 [9].
In parallel, extracorporeal support for other organs was developed. Gibbon was the first to use artificial oxygenation and perfusion support for the first successful open-heart surgery in 1953 [10]. Ten years later, Kolobow described the construction and evaluation of an alveolar membrane artificial heart lung [11]. This was “the embryo” of the extracorporeal membrane oxygenation (ECMO), which was first successfully used in treatment by Hill in 1972.
Based on this previous experience, liver-support therapies using albumin dialysis as principle, and CO2 removal devices employing membrane oxygenators are now available. Moreover, other add-on devices (e.g. CytoSorb) for the removal of disease mediators during sepsis have also gained attention.
This shows a large battery of therapies available. However, as suggested by other authors [4,5,6], it is expected that future developments converge into a single device capable of achieving multiorgan support to cover the lung, the heart, the kidney and the liver [5]. In line with this, a landmark animal study characterized already more than 30 years ago the potential hemodynamic impairment as well as the amount of blood flow required for renal replacement, decarboxylation and oxygenation (Table 1; [12]).
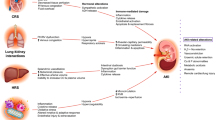
Driven by the “proof of principle” of long-term organ support by chronic hemodialysis, numerous devices for extracorporeal single organ support have been introduced (Fig. 1).
Despite specific features these devices share some common principles and risks (Table 2).
Characteristics of specific organ support
Renal replacement
Up to 7% of hospitalized patients develop acute kidney injury [13] during their hospital stay. Among critically ill patients in the intensive care unit (ICU), this rate reaches even 25% [14]. What is more, a mortality rate >50% has been reported for patients with AKI and multiorgan failure [15]. In the absence of any effective pharmacologic therapies, severe AKI can only effectively be managed by RRT.
RRT can be applied with continuous or intermittent modalities. On the one hand, continuous renal replacement therapy (CRRT) refers to any device or technique aiming to replace kidney function for blood purification during an extended period of time. Intermittent therapies are conducted during up to 5 h. A successful CRRT results in a better hemodynamic stability, reduced transcellular solute shifts, and better tolerance to fluid removal. On the contrary, the need of continuous anticoagulation, patient monitoring, alarm vigilance, and experienced staff can be seen as its major disadvantages. On the other hand, during intermittent treatments, an adequate vascular access, specially trained nurses, and continuous pure water supply are demanded. Several forms of RRT can be employed [16]:
-
Slow continuous ultrafiltration (SCUF) is a continuous therapy that might be used to reach a correction of fluid overload in refractory patients by applying a slow removal of plasma water.
-
Continuous veno-venous hemofiltration (CVVH) provides solute clearance and volume control by convection. Replacement fluids are infused before or after the hemofilter to replace the ultrafiltrate by predilution or postdilution, respectively.
-
Continuous veno-venous hemodialysis (CVVHD) uses diffusion for detoxification. This is achieved flowing dialysate into the dialysate compartment of the hemodialyzer either co-currently or counter-currently. IHD refers to intermittent hemodialysis.
-
Continuous veno-venous hemodiafiltration (CVVHDF) is a combination of the two previous techniques. The intermittent variant is known as intermittent hemodiafiltration (IHDF),
-
Continuous veno-venous high-flux hemodialysis (CVVHFD) or intermittent high-flux dialysis (IHFD) is a modified hemodialysis where high-flux membranes are applied.
Extracorporeal lung support: oxygenation
Despite several effective approaches including prone positioning and low tidal volume ventilation, acute respiratory distress syndrome [17] still has a mortality of more than 40% and affects about 10% of ICU patients. Extracorporeal lung support was introduced more than 80 years ago with Gibbon’s heart–lung machine [18]. The first case reports on the clinical use of ECMO in ARDS and preterm infants were published in the 1970s. The first two randomized controlled trials (RCTs) provided the proof of principle with improved oxygenation, but no survival benefit. The lack of improved outcome was mainly due to unacceptably high blood losses and the absence of a lung-protective ventilation under ECMO [19, 20]. Heparin-coating of the ECMO surfaces allowed for a reduction of high-dose heparinization and reduced complication rates in the two more recent RCTs: CESAR and EOLIA [21, 22]. Both trials gave hints on a reduction of mortality by ECMO in selected patients with ARDS. Nevertheless, the improvement of the outcome was lower than assumed for the power calculation in both trials. In fact, the EOLIA trial was stopped for futility despite a nonsignificant 11% reduction in mortality. Both studies and several registries provided important subgroup analyses suggesting several approaches to improve the effect size of ECMO. Among those are a better patient selection and an optimized set-up of the extracorporeal device. Patients with ARDS should be allocated early (i.e. within about 4 days of intubation). Subtle subgroup analyses of EOLIA suggest that ECMO was more beneficial in patients with less impairment of oxygenation (pO2/FiO2 ≥66 mm Hg), but more pronounced hypercapnia (pCO2 ≥55 mm Hg).
Furthermore, outcome of patients with ECMO therapy is strongly predicted by concomitant nonpulmonary organ failure. In EOLIA, ECMO reduced mortality from 39 to 22% in patients with a SOFA score <11 but was completely ineffective in patients with SOFA ≥11.
This emphasizes the need for improved multiorgan support. Interestingly, 17% of the patients randomized to ECMO in the CESAR trial (but none of the controls) were treated with the MARS liver support device.
Extracorporeal lung support: CO2 removal
Considering the invasiveness and risks of high-flow ECMO, Gattinoni and coworkers introduced the concept of less invasive extracorporeal lung support restricted to CO2 removal (ECCO2R) [23].
With a more limited blood flow, ECCO2R technologies are intriguing for combination with other ECOS devices, in particular with RRT. As shown in Table 3, at least five studies reported on the feasibility of low-flow ECCO2R combined with an ultraprotective ventilation aimed at tidal volumes of 4 instead of 6 ml/kg predicted bodyweight (Table 3).
Finally, pumpless extracorporeal lung assist (pECLA) with a blood flow around 1000 ml/min has been shown to effectively remove CO2, while improvement of oxygenation is limited due to the “midrange” blood flow [24, 25].
Regarding multiorgan support, some of the ECCO2R devices are prepared for combined use with CVVH(D)F. However, most of these studies (Table 3) excluded patients with other organ failures (in particular liver failure). By contrast, the ongoing ADVOPROTECT trial deliberately includes patients with liver and renal failure.
Another technology of interest has been termed “respiratory electrodialysis”. This procedure combines a hemodiafilter with a membrane lung and a electrodialysis cell cell positioned on the hemodiafiltrate. This technology regionally increases the blood chloride concentration to convert bicarbonate to CO2, thus enhancing the CO2 extraction by the membrane lung [26, 27].
Extracorporeal liver support
In addition to the kidneys and lungs, the liver is one of three major detoxification organs. While renal failure results in the accumulation of water-soluble toxins and fluid, liver failure reduces the elimination of protein-bound toxins and liver synthesis.
During the 1990s several extracorporeal methods to eliminate protein-bound toxins were introduced. The most common approach to date is termed albumin dialysis. It is based on the addition of 2–6% albumin to the dialysate to facilitate transport of protein-bound toxins from the blood across the semipermeable membrane into the dialysate. Single-pass albumin dialysis (SPAD) is straightforward but results in a complete waste of the albumin- and toxin-containing dialysate. The proof of principle has been shown in a patient with a serum bilirubin concentration of 102 mg/dL due to liver failure induced by Wilson disease [32]. Although the method is effective for bilirubin and copper removal, the albumin waste results in inacceptable financial burden, particularly, in case of repeated treatment. Therefore, several approaches to “regenerate” the toxin-loaded albumin in the dialysate have been introduced.
MARS.
The molecular adsorbent recirculating system [33] has been shown to efficiently remove bilirubin as well as ammonia and creatinine. The toxin-loaded albumin in the dialysate is regenerated in a secondary circuit with two adsorption columns (charcoal and an anion-exchange resin). Initial clinical trials suggest improvement of encephalopathy, circulation, portal hypertension and major outcomes. Nevertheless, the largest RCT, the RELIEF trial [34], did not show overall improvement of survival of patients with acute on chronic liver failure (ACLF) [34]. However, a recent subgroup analysis demonstrated an improved 28-day transplant-free survival of patients with ACLF grade two or three [35]. According to the ACLF definition, these were the more severely ill subgroups with at least two or three organ failures. This suggests a potential of MARS for multiorgan support by elimination of water- and protein-bound toxins.
Fractionized plasma separation and adsorption system (FPSA; Prometheus).
This technology combines separation of toxin-loaded albumin by an albumin-permeable membrane, and removal of the protein-bound toxins through two absorbers (a neutral resin and an anion exchanger) with hemodialysis once the purified plasma returns to the extracorporeal blood circuit. Similar to the RELIEF trial with MARS, also the HELIOS trial with the Prometheus device did not show improvement in survival by extracorporeal FPSA therapy. However,—again—there was a significant survival benefit for the more severely ill patients of the subgroup with a MELD score >30 [36].
High-volume plasma exchange (HVP).
Plasma separation and replacement with fresh-frozen plasma (FFP) is an established extracorporeal procedure for removing protein-bound toxins. Furthermore, it allows for efficient support of plasmatic coagulation. Several smaller case series gave hints that HVP might improve the outcome in patients with acute liver failure (ALF). A RCT comprising 182 patients with ALF demonstrated significantly improved survival and a significant reduction in the SOFA score and SIRS criteria by HVP [37]. Interestingly, the survival benefit of HVP was greater in those patients who did not undergo emergency liver-transplantation.
Bioartificial liver (BAL) support.
Extracorporeal bioartificial cellular therapies using extracorporeal liver cell bioreactors for blood purification have been investigated for decades. However, results in patients are still controversial. A recent meta-analysis on 18 clinical trials and 12 preclinical studies, suggested survival improvements are only shown in large animals, but not in humans with ALF [38]. In order to see progress in this area, alternative high-quality liver cells might be necessary, together with well-designed trials, analyzing the effects on subgroups such as primary nonfunction or fulminant hepatic failure. A phase 2 study did not show improved outcome of patients with end-stage liver disease, but demonstrated a trend to better outcome in a subgroup of patients with alcoholic steatohepatitis [39]. A RCT with 203 patients did not demonstrate an improved overall survival in patients treated with the extracorporeal liver assist device (ELAD) compared to standard therapy. Subgroup analyses suggest a potential benefit in younger patients (<47 years) with a MELD score <28 [39].
Hemadsorption.
A few case reports and small case-series suggest that bilirubin is eliminated by the hemadsorption device CytoSorb [40]. Based on the methodology, so far no conclusions about an improved outcome can be drawn so far.
Advanced organ support
The advanced organ support (ADVOS) multihemodialysis device is based on the principle of albumin dialysis. The proof of principle has been shown in preclinical studies and case series [41,42,43]. Beyond the normal renal replacement function, it can eliminate protein-bound substances and CO2 [44]. These properties are due to an “intelligent” dialysate: Toxins diffused from blood into the dialysate are eliminated after the application of physicochemical changes (e.g., pH) to the recirculating dialysate in a secondary circuit. This is due to conformational change occurring in albumin above a concrete pH level, which helps both to toxin removal and albumin recycling [13]. In addition, since the dialysate is formed via the on-line mixing of an acidic and an alkaline concentrate, the previously mentioned pH changes can be customized to adapt the dialysate pH. Overall, ADVOS intends to provide a multiple organ (i.e. kidney, liver, lungs) support by means of water-soluble, protein-bound toxins elimination, direct H+ removal (i.e. acid–base balance) and CO2 elimination.
Serum albumin, is the main protein of human blood plasma. It binds, among others, fatty acids, hormones or bilirubin. An increase of the latter 5 times above the upper limit increases the risk to develop cholemic nephropathy [45,46,47]. Furthermore, new onset of acute kidney injury is associated with concomitant onset of jaundice [48]. The reduction of bilirubin levels (ideally by normalization of the hepatic function, alternatively by extracorporeal detoxification) by the ADVOS multi device has been shown in several studies. On top of this, as already documented [43], ADVOS multi can remove creatinine, urea or ammonia, among others.
Nevertheless, probably, the most differentiating factor of the ADVOS therapy in comparison to other apparently similar medical devices is the possibility to adjust the pH of the dialysate (by the relation between the acidic and basic concentrates that form the dialysate) and adapt it to the needs of the patient during treatment. Going back to chemistry basics, when the pH of a solution is higher than 7.00, the concentration of OH− is likewise higher than that of H+. The higher the pH of the dialysate, the higher the gradient of H+ that can be formed between blood and dialysate. Consequently, H+ in excess will diffuse from blood into the dialysate, providing an acidosis correction. Moreover, by removing H+, HCO3− will be produced in blood (Eq. 1), mimicking the mechanism used by the kidney as a metabolic response to respiratory acidosis.
Equation 1.
Equilibrium reaction between CO2, H+ and HCO3−
The generated HCO3− provides an improvement during metabolic acidosis, but should be removed, if excessive, during respiratory acidosis. The capacity of the ADVOS system to remove CO2 depends on blood flow, dialysate pH and the bicarbonate concentration. As demonstrated in a series of experiments using an ex vivo model for acidosis, the higher the dialysate pH, the blood flow or the accumulated HCO3−, the better CO2 removal rates are achieved [44]. In the clinical setting ADVOS is normally used with a maximum blood flow rate of 200 ml/min (to allow regional citrate anticoagulation), a maximum dialysate pH of 9 and basic concentrates containing 20 mmol/l HCO3−. This allows a removal of up to 50 ml/min CO2 with normal blood bicarbonate concentration (22–28 mmol/l). Since the HCO3− removal is the limiting factor in the ADVOS multi circuit, during a severe metabolic acidosis even more CO2 could be removed without an increase of blood bicarbonate over 30 mmol/l. Under experimental conditions, up to 146 ml/min of CO2 could be removed. However, this required blood flow rates of 400 ml/min and a dialysate pH >9.00 with a basic concentrate without bicarbonate [44].
In contrast to ECMO, where due to high blood flows (3–6 L/min) blood pH is normalized within minutes, it takes up to 2–4 h for ADVOS multi running at 100–200 ml/min blood flows until an acidotic blood is normalized in patients. The use of elevated dialysate pH is not exempt of risks, and therefore, to avoid overcompensation, blood pH must be continuously monitored during ADVOS treatments. It is recommended that blood pH values of the samples taken at the outlet of the dialyzer (blood post-dialyzer) remain below 8.00. Above this value pH is no longer measurable in common blood gas analyzers. In case that a post-dialyzer blood pH is >8.00, dialysate pH should be reduced by 0.5 in the treatment’s settings (e.g., from 9.00 to 8.50).
Table 4 summarizes the main features of clinically available devices for extracorporeal liver support.
Detoxification in sepsis
Major parts of the pathophysiology of sepsis are related to microbial toxins and to the inflammatory response induced by proinflammatory cytokines. Therefore, extracorporeal elimination of toxins and cytokines is an intriguing concept to treat patients with sepsis.
In the first case, hemoperfusion using fiber columns containing polymyxin B (an antibiotic with high affinity to endotoxins) has been used in a number of studies. However, recent results and meta-analyses did not demonstrate improved outcome by this or similar approaches [33, 49,50,51].
In the second case, CytoSorb provides hemoadsorption of cytokines and other midmolecular weight toxins by multiple porous polymeric beads. Two larger studies in septic patients resulted in conflicting data: A RCT including 100 mechanically ventilated patients with severe sepsis or septic shock did no show a reduction in systemic IL‑6 levels or in multiple organ dysfunction score, ventilation time and time course of oxygenation in the intervention group [52]. A retrospective analysis of 116 patients with septic shock demonstrated a significantly higher reduction in predicted mortality in patients with CytoSorb therapy and CRRT compared to patients with CRRT alone [53].
Similarly, the HA 330 and HA 380 cartridges (Jafron, Zhuhai, China) contain neutro-macroporous resin adsorbing beads with a pore size of 500 D–60 kD. At least two RCTs with 44 and 46 patients demonstrated significantly improved outcome (including ICU mortality) in patients treated with HA 330 hemoperfusion [54, 55].
Modular or integrated multiorgan support?
While there is increasing evidence for combined MOST, there is an ongoing debate about its realization. From a pragmatic viewpoint individual combination of the available devices is a first reasonable step. In particular, liver support systems such as MARS and Prometheus, and some devices for ECCO2R are usually combined with sequential RRT devices. Furthermore, the high blood flow during ECMO allows for RRT in parallel without additional vascular access [56].
Nevertheless, modular combination results in additional extracorporeal volume and potential hemodynamic impairment. Also regarding fluid balance targets, thorough monitoring of these side effects is mandatory. This starts with the observation of potential hemodynamic impairment during connection and ends with documentation of circulatory changes during disconnection. Several studies showed that transpulmonary thermodilution (TPTD) is feasible during RRT and ADVOS treatments [56]. Despite concerns on a loss of indicator into the extracorporeal circuit, a recent study demonstrated that measurement of Cardiac Index with TPTD is reliable even during ECMO [57], whereas global end-diastolic volume index (GEDVI) and extravascular lung water index (EVLWI) might be confounded.
Regarding the disadvantages and technical burdens of using combinations of pre-existing technologies (Table 5), development of procedures facilitating MOST by one single device is an intriguing next step. Although there is still a lack of data on improved outcome, ADVOS can be considered as the first integrated MOST device.
Practical conclusion
During the last few decades, extracorporeal organ support has become available for nearly every organ failure. All types of ECOS share the challenges of vascular access, sequestration of blood into the device, induction of extracorporeal blood flow, anticoagulation with potential bleeding or clotting complications, a certain circulatory impairment, and finally, the attempt of extracorporeal blood purification.
Based on organ-specific compensatory mechanisms and blood flow within the genuine organ(s), extracorporeal blood flow ranges from below 100 ml/min up to more than 5 l/min in ECMO. Due to the high incidence of MOF in critically ill patients, the concept of multiorgan support is intriguing. Depending on the individual organ failures, in some patients, multiorgan support can be provided by sequential and/or intermittent therapy with single-organ support systems. Another option is combined organ support (normally two organ support) using serially connected devices driven by one blood pump. Considering the additive sequestration of blood in several devices, integrated multiorgan support using one multifunctional device might be the most intriguing approach.
References
Skillman JJ et al (1969) Respiratory failure, hypotension, sepsis, and jaundice. A clinical syndrome associated with lethal hemorrhage from acute stress ulceration of the stomach. Am J Surg 117(4):523–530
Ronco C, Bellomo R (2002) Acute renal failure and multiple organ dysfunction in the ICU: from renal replacement therapy (RRT) to multiple organ support therapy (MOST). Int J Artif Organs 25(8):733–747
Husain-Syed F et al (2018) Extracorporeal organ support (ECOS) in critical illness and acute kidney injury: from native to artificial organ crosstalk. Intensive Care Med 44(9):1447–1459
Ronco C, Ricci Z, Husain-Syed F (2019) From multiple organ support therapy to extracorporeal organ support in critically ill patients. Blood Purif 48(2):99–105
Ranieri VM, Brodie D, Vincent JL (2017) Extracorporeal organ support: from technological tool to clinical strategy supporting severe organ failure. JAMA 318(12):1105–1106
Vincent JL (2019) Introduction to extracorporeal multiple organ support. Blood Purif 48(2):97–98
Vincent JL et al (1998) Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European society of intensive care medicine. Crit Care Med 26(11):1793–1800
Gottschalk CW, Fellner SK (1997) History of the science of dialysis. Am J Nephrol 17(3–4):289–298
Twardowski ZJ (2008) History of hemodialyzers’ designs. Hemodial Int 12(2):173–210
Boettcher W, Merkle F, Weitkemper HH (2003) History of extracorporeal circulation: the conceptional and developmental period. J Extra Corpor Technol 35(3):172–183
Kolobow T, Bowman RL (1963) Construction and evaluation of an alveolar membrane artificial heart-lung. Trans Am Soc Artif Intern Organs 9:238–243
Gattinoni L et al (1978) Control of intermittent positive pressure breathing (IPPB) by extracorporeal removal of carbon dioxide. Br J Anaesth 50(8):753–758
Yamasaki K et al (1999) Interactive binding to the two principal ligand binding sites of human serum albumin: effect of the neutral-to-base transition. Biochim Biophys Acta 1432(2):313–323
Tolwani A (2012) Continuous renal-replacement therapy for acute kidney injury. N Engl J Med 367(26):2505–2514
Uchino S et al (2005) Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294(7):813–818
Villa G et al (2016) Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical applications. Crit Care 20(1):283
Combes A et al (2019) Feasibility and safety of extracorporeal CO2 removal to enhance protective ventilation in acute respiratory distress syndrome: the SUPERNOVA study. Intensive Care Med 45(5):592–600
Passaroni AC, Silva MA, Yoshida WB (2015) Cardiopulmonary bypass: development of John Gibbon’s heart-lung machine. Rev Bras Cir Cardiovasc 30(2):235–245
Morris AH et al (1994) Randomized clinical trial of pressure-controlled inverse ratio ventilation and extracorporeal CO2 removal for adult respiratory distress syndrome. Am J Respir Crit Care Med 149(2 Pt 1):295–305
Zapol WM et al (1979) Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA 242(20):2193–2196
Combes A, Slutsky AS, Brodie D (2018) ECMO for severe acute respiratory distress syndrome. N Engl J Med 379(11):1091–1092
Peek GJ et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374(9698):1351–1363
Gattinoni L et al (1986) Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 256(7):881–886
Bein T et al (2013) Lower tidal volume strategy (approximately 3 ml/kg) combined with extracorporeal CO2 removal versus ‘conventional’ protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 39(5):847–856
Kluge S et al (2012) Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 38(10):1632–1639
Zanella A et al (2019) Extracorporeal chloride removal by electrodialysis (Cre-ED): a novel approach to correct acidemia. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.201903-0538OC
Zanella A et al (2015) Respiratory electrodialysis. A novel, highly efficient extracorporeal CO2 removal technique. Am J Respir Crit Care Med 192(6):719–726
Terragni PP et al (2009) Tidal volume lower than 6 ml/kg enhances lung protection: role of extracorporeal carbon dioxide removal. Anesthesiology 111(4):826–835
Fanelli V et al (2016) Feasibility and safety of low-flow extracorporeal carbon dioxide removal to facilitate ultra-protective ventilation in patients with moderate acute respiratory distress sindrome. Crit Care 20:36
Schmidt M et al (2018) Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care 22(1):122
Nentwich J et al (2019) Low-flow CO2 removal in combination with renal replacement therapy effectively reduces ventilation requirements in hypercapnic patients: a pilot study. Ann Intensive Care 9(1):3
Kreymann B et al (1999) Albumin dialysis: effective removal of copper in a patient with fulminant Wilson disease and successful bridging to liver transplantation: a new possibility for the elimination of protein-bound toxins. J Hepatol 31(6):1080–1085
Dellinger RP et al (2018) Effect of targeted polymyxin B hemoperfusion on 28-day mortality in patients with septic shock and elevated endotoxin level: the EUPHRATES randomized clinical trial. JAMA 320(14):1455–1463
Banares R et al (2013) Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: the RELIEF trial. Hepatology 57(3):1153–1162
Gerth HU et al (2017) Molecular adsorbent recirculating system can reduce short-term mortality among patients with acute-on-chronic liver failure—a retrospective analysis. Crit Care Med 45(10):1616–1624
Kribben A et al (2012) Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 142(4):782–789.e3
Larsen FS et al (2016) High-volume plasma exchange in patients with acute liver failure: an open randomised controlled trial. J Hepatol 64(1):69–78
He YT et al (2019) Bioartificial liver support systems for acute liver failure: a systematic review and meta-analysis of the clinical and preclinical literature. World J Gastroenterol 25(27):3634–3648
Thompson J et al (2018) Extracorporeal cellular therapy (ELAD) in severe alcoholic hepatitis: a multinational, prospective, controlled, randomized trial. Liver Transpl 24(3):380–393
Dhokia VD et al (2019) Novel use of cytosorb haemadsorption to provide biochemical control in liver impairment. J Intensive Care Soc 20(2):174–181
Al-Chalabi A et al (2013) Evaluation of the hepa wash(R) treatment in pigs with acute liver failure. BMC Gastroenterol 13:83
Al-Chalabi A et al (2017) Evaluation of an ADVanced organ support (ADVOS) system in a two-hit porcine model of liver failure plus endotoxemia. Intensive Care Med Exp 5(1):31
Huber W et al (2017) First clinical experience in 14 patients treated with ADVOS: a study on feasibility, safety and efficacy of a new type of albumin dialysis. BMC Gastroenterol 17(1):32
Perez Ruiz de Garibay A et al (2019) Respiratory and metabolic acidosis correction with the ADVanced organ support system. Intensive Care Med Exp 7(1):56
Brasen JH et al (2019) Cholemic nephropathy causes acute kidney injury and is accompanied by loss of aquaporin 2 in collecting ducts. Hepatology 69(5):2107–2119
Foshat M et al (2017) Bile cast nephropathy in cirrhotic patients: effects of chronic hyperbilirubinemia. Am J Clin Pathol 147(5):525–535
Nayak SL et al (2017) Bile cast nephropathy in patients with acute kidney injury due to hepatorenal syndrome: a postmortem kidney biopsy study. J Clin Transl Hepatol 5(2):92–100
Jager B et al (2012) Jaundice increases the rate of complications and one-year mortality in patients with hypoxic hepatitis. Hepatology 56(6):2297–2304
Fujii T et al (2018) Polymyxin B‑immobilized hemoperfusion and mortality in critically ill adult patients with sepsis/septic shock: a systematic review with meta-analysis and trial sequential analysis. Intensive Care Med 44(2):167–178
Broman ME et al (2019) Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: a randomized crossover double-blind study. PLoS ONE 14(8):e220444
Umgelter A et al (2008) Treatment of septic patients with an arginine-based endotoxin adsorber column improves hemodynamics and reduces oxidative stress: results of a feasibility study. Blood Purif 26(4):333–339
Schadler D et al (2017) The effect of a novel extracorporeal cytokine hemoadsorption device on IL‑6 elimination in septic patients: a randomized controlled trial. PLoS ONE 12(10):e187015
Brouwer WP et al (2019) Hemoadsorption with CytoSorb shows a decreased observed versus expected 28-day all-cause mortality in ICU patients with septic shock: a propensity-score-weighted retrospective study. Crit Care 23(1):317
Huang Z et al (2010) Removal of humoral mediators and the effect on the survival of septic patients by hemoperfusion with neutral microporous resin column. Ther Apher Dial 14(6):596–602
Huang Z et al (2013) Effect on extrapulmonary sepsis-induced acute lung injury by hemoperfusion with neutral microporous resin column. Ther Apher Dial 17(4):454–461
Lahmer T et al (2017) In-parallel connected intermittent hemodialysis through ECMO does not affect hemodynamic parameters derived from transpulmonary thermodilution. Perfusion 32(8):702–705
Herner A et al (2019) Transpulmonary thermodilution before and during veno-venous extra-corporeal membrane oxygenation ECMO: an observational study on a potential loss of indicator into the extra-corporeal circuit. J Clin Monit Comput. https://doi.org/10.1007/s10877-019-00398-6
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
W. Huber is member of the Medical Advisory Board of Pulsion Medical systems SE (Getinge Group). W. Huber received speaker’s fees and travel reimbursement by ADVITOS GmbH. W. Huber is principal investigator of a clinical ECMO study supported by NovaLung/Xenios (Fresenius Medical Care). W. Huber is principal investigator of an animal study on ECMO and hemodynamics supported by Maquet GmbH (Getinge Group). A.P. Ruiz de Garibay is in an employment relationship with ADVITOS GmbH.
For this article no studies with human participants or animals were performed by any of the authors. All studies performed were in accordance with the ethical standards indicated in each case.
The supplement containing this article is not sponsored by industry.
Additional information
Redaktion
M. Bauer; Jena
M. Singer, London
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huber, W., Ruiz de Garibay, A.P. Options in extracorporeal support of multiple organ failure. Med Klin Intensivmed Notfmed 115 (Suppl 1), 28–36 (2020). https://doi.org/10.1007/s00063-020-00658-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00063-020-00658-3
Keywords
- Extracorporeal organ support
- Renal replacement therapy
- Albumin dialysis
- Plasma separation
- Extracorporeal CO2 removal