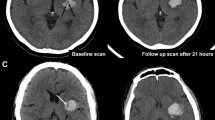
Abstract
Purpose
Intracerebral hemorrhage is the deadliest form of stroke. This study aimed to enhance the prediction of 30-day mortality in intracerebral hemorrhage patients by integrating computational parameters.
Methods
This study retrospectively analyzed 435 patients with spontaneous intracerebral hemorrhage (ICH). Utilizing the acquired computed tomography (CT) images, we extracted the contour and visual representation of ICH. For the extracted contour, the analysis encompassed factors including compactness, fractal dimension, Fourier factor, and circle factor. For the images depicting ICH, we calculated various factors related to density distribution including mean, coefficient of variance, skewness and kurtosis, as well as texture parameters, such as energy, entropy, contrast and homogeneity. To assess the impact of surgical treatment on 30-day mortality, logistic regression analysis was used.
Results
A total of 126 patients (29.09%) died within 30 days. A total of 62 (14.25%) patients underwent surgical treatment. Multivariate logistic regression analysis revealed that surgical treatment was independently associated with a lower risk of 30-day mortality (odds ratio, OR 0.226, 95% confidence interval, CI 0.049–0.85; p = 0.039). Based on the moderated analysis, we found that the volume of ICH (OR 0.905, 95% CI 0.902–0.908; p < 0.001) and ICH energy (OR 1.389, 95%CI 0.884–0.988; p = 0.010) had positive moderating effect on such associations while the presence of intraventricular blood had negative moderating effect (OR 1.154, 95% CI 1.034–1.628; p = 0.010).
Conclusion
Patients exhibiting a higher volume and energy of ICH might benefit from surgical treatment; however, this efficacy was found to be diminished in cases involving the presence of intraventricular blood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (ICH) accounts for 10–30% of all acute strokes [1] and is characterized by the highest mortality rate compared to all other subtypes [2]. Additionally, it is associated with a high percentage of functional dependence [3]. Therefore, the exploration of potential treatment strategies is currently the subject of extensive research. Particular attention has been given to investigating the potential advantages of surgical interventions in the treatment of ICH. The choice of surgical management in ICH is understood to be associated with several benefits, such as reduction of the mass effect of ICH and minimizing the impact of toxic blood components on brain tissue [4]. Although meta-analyses and reviews suggested a positive impact of surgical treatment on mortality [5], the results of clinical trials in this matter remain inconclusive. The first randomized, controlled trial, surgical treatment for intracerebral hemorrhage (STICH), failed to demonstrate the benefits of surgically removing ICH; however, a subsequent study STICH II, suggested that surgical treatment of lobar hematomas might improve outcomes [7]. Further trials focusing on minimally invasive techniques for ICH removal have also suggested the potential benefit of such procedures [8, 9]. The American Heart Association guidelines state that the efficacy of surgical treatment for ICH is inconclusive and recommended surgical intervention for patients with cerebellar hemorrhage and neurological deterioration or for those suffering from brainstem compression [10]. Predictors of poor outcome following ICH have also been extensively studied. Radiomics analysis of ICH imaging has shown potential correlations with mortality and risk of hemorrhage enlargement [11,12,13]; however, there is a lack of research exploring the potential association between radiomics analysis and outcomes following surgical treatment of ICH. Consequently, our study aims to re-examine potential predictors of 30-day mortality post-ICH with particular emphasis on the implications of surgical treatment and ICH appearance on computed tomography (CT) imaging.
Methods
Study Group
We retrospectively analyzed patients hospitalized in the Department of Neurology or in the Department of Neurosurgery of the University Hospital in Krakow between January 2013 and October 2020, who met the following criteria: (1) had first spontaneous ICH diagnosed based on head CT, (2) presence of intracranial aneurysm or other vascular abnormalities was excluded based on angio-CT or digital subtraction angiography, (3) had no other intracranial pathologies including tumors, (4) had no previous neurosurgical or endovascular procedures and (5) had no history of spontaneous ICH. From the medical records we obtained data such as current diseases and medications, blood tests results taken < 4 h following admission and Glasgow coma scale (GCS) score taken on admission. We also obtained the CT images taken < 4 h after admission. Based on these images we assessed parameters such as ICH location, volume and presence of intraventricular hemorrhage (IVH). For study group we assessed 30-day mortality.
Computational Analysis of ICH Appearance
The authors developed a software written in Python and performed a series of transformations on CT images obtained from each patient. Each image was represented as a two-dimensional pixel array with density values between 0 and 255. Then we semiautomatically extracted the images containing ICH, image of 4 mm thickness brain tissue surrounding ICH, defined as the perihemorrhage area (PHA) and 20 × 20 pixel image of healthy brain tissue for reference (Fig. 1). Additionally, we extracted a curve representing contours of the ICH. For the extracted images of ICH and PHA we analyzed the distribution of density values by calculating its mean, coefficient of variance (mean/standard deviation), skewness and kurtosis. Additionally, we computed these parameters for the brain tissue sample. The presented ICH and PHA density distribution values are normalized to healthy brain tissue. Furthermore, we conducted texture analysis by constructing a gray level co-occurrence matrix (matrix with distribution of co-occurring pixel values at a given offset) [14]:
where i and j are pixel values, x and y are the spatial positions in the n × m size image I and (∆x, ∆y) is the given offset. For each image we constructed four matrices with offset d = (2.2) in directions of 0°, 45°, 90° and 135°. Based on matrices we calculated the following descriptors:
For extracted contours we calculated the following parameters: fractal dimension, Fourier factor, circle factor and compactness. Details about contour parameters and used software were presented in our previous research [15].
We used the Shapiro-Wilk test to assess normality. For continuous variables that followed a normal distribution, we utilized the t‑test and for those variables which were not normally distributed, we performed the Mann-Whitney U-test. We used the χ2 test for proportional variables. We expressed continuous variables as mean ± standard deviation. To analyze independent predictors of 30-day mortality after ICH we used multivariate logistic regression analysis. Additionally, we employed logistic regression analysis to assess the factors moderating effect of surgical treatment on 30-day mortality. To identify the cut-off points for moderating the effects of ICH energy and volume, Receiver Operating Characteristic (ROC) curve analysis was utilized. P‑values < 0.05 were considered statistically significant. All statistical analyses and the preparation of figures were conducted using RStudio version 8.5 for Windows (Posit, Boston, MA, USA).
Results
Univariate and Multivariate Analyses
A total of 435 patients met the inclusion criteria. Detailed characteristics of studied groups are presented in Table 1. Of the patients 126 (29.09%) died within the first 30 days and 62 patients (14.25%) underwent surgical treatment. Patients who underwent surgical treatment had significantly lower GCS score upon admission (9.11 ± 4.84 vs. 10.81 ± 4.24; p < 0.01), significantly less often had hypertension (30.65% vs. 52.02%; p < 0.01) and diabetes mellitus (4.84% vs. 17.52%; p = 0.01). Patients receiving surgical treatment were also significantly more likely to have ICH in the temporal lobe (22.58% vs. 12.94%; p = 0.045) or cerebellum (24.19% vs. 9.97%; p < 0.01) and significantly less frequently in the basal ganglia (22.58% vs. 36.93%; p = 0.03). Regarding the CT appearance of ICH, these patients exhibited significantly higher compactness (0.66 ± 0.22 vs. 0.58 ± 0.2; p < 0.01), energy (0.23 ± 1.31 vs. 0.04 ± 0.93; p = 0.048), Contrast (0.31 ± 1.21 vs. 0.05 ± 0.95; p < 0.01), and Homogeneity (0.32 ± 1.09 vs. 0.05 ± 0.97; p < 0.01), but significantly lower Fourier Factor (0.82 ± 0.09 vs. 0.86 ± 0.07; p < 0.01) and Circle Factor (0.35 ± 0.15 vs. 0.41 ± 0.15; p < 0.01) (Table 1). Multivariate analysis of possible predictors of 30-day mortality (Table 2), performed for entire study group, revealed that surgical treatment was independently associated with lower 30-day mortality (OR:0.226; 95%CI:0.049–0.85; p = 0.039).
Moderation Effect of ICH Energy
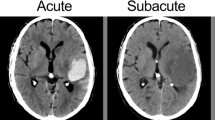
Our findings indicate a significant positive moderation by ICH energy on the reduction of 30-day mortality following surgical treatment (OR: 0.932; 95% CI: 0.884–0.988; p = 0.01) (Table 2). To distinguish between patients with low and high nergye, we established the cut-off point for such moderation at 27.92 (p < 0.01). Examples of ICH with high and low energy are presented in Fig. 2. Among patients above this threshold (54.50%) surgical treatment was independently associated with a lower risk of 30-day mortality (OR: 0.113; 95% CI: 0.015–0.593; p = 0.02). Additionally, a lower GCS score upon admission (OR: 0.731; 95% CI: 0.632–0.83; p < 0.01), a larger volume of ICH (OR: 1.26; 95% CI: 1.021–1.53; p = 0.043), higher glucose levels at admission (OR: 1.246; 95% CI: 1.091–1.476; p < 0.01), and the presence of IVH (OR: 3.177; 95% CI: 1.366–7.769) were independently associated with an increased risk of 30-day mortality. Below determined threshold (45.50%) only older age (OR: 1.068; 95% CI: 1.025–1.120; p < 0.01) and the subtentorial location (OR: 1.020; 95% CI: 1.003–1.041; p = 0.031) were independently associated with higher risk of 30-day mortality (Table 3, Fig. 3).
Moderation Effect of ICH Volume
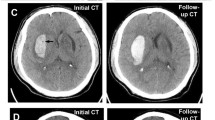
Furthermore, we found a significant positive moderation by ICH volume on the reduction of 30-day mortality following surgical treatment (OR: 0.905; 95% CI: 0.902–0.908; p < 0.01). To distinguish between patients with low and high volumes, we established cut-off point for volume moderation at 29.47 ml (p < 0.01). Among patients above that threshold (49.19%), surgical treatment was associated with lower risk of 30-day mortality (OR: 0.037; 95% CI: 0.001–0.414; p = 0.03), a higher GCS score upon admission (OR: 0.764; 95% CI: 0.657–0.872; p < 0.01) and a higher entropy (OR: 0.994; 95% CI: 0.986–0.998; p = 0.04). A higher glucose level upon admission (OR: 1.22; 95% CI: 1.063–1.443; p < 0.01), presence of IVH (OR: 3.979; 95% CI: 1.495–11.401; p < 0.01) and a higher fractal dimension (OR: 1.489; 95% CI: 1.114–2.091; p = 0.01) were independently associated with a higher risk of 30-day mortality among those patients. In terms of patients with lower ICH volume (50.81%) higher GCS score (OR: 0.570; 9%% CI: 0.435–0.695; p < 0.01) and subtentorial location (OR: 0.035; 95% CI: 0.001–0.442; p = 0.02) were independently associated with lower risk of 30-day mortality and higher energy was associated with higher risk of 30-day mortality (OR: 1.419; 95% CI: 1.175–2.302; p = 0.03) (Table 4, Fig. 4).
Discussion
Our study revealed that the majority of patients received conservative treatment, which is consistent with the commonly adopted management approach [10]. Surgically treated patients exhibited significantly fewer comorbidities, despite being in a worse neurological state. Moreover, surgical treatment independently contributed to a reduction in 30-day mortality. As previously mentioned, although the STICH trial failed to demonstrate the benefits of surgical treatment for of ICH [6], further analysis revealed that this approach may enhance outcomes for certain patient groups [7, 9]. Similarly, our study identified factors that could moderate the impact of ICH removal on mortality, which should be considered in management planning.
We uncovered significant differences in the CT appearance of surgically treated ICH. Based on existing literature, ICH radiomics have not been analyzed for surgical treatment indications; however, it’s association with outcomes and the risk of ICH growth has been demonstrated [11,12,13]. This aligns with our findings that energy and entropy were independently linked to an increased risk of 30-day mortality in our study group. Another interesting finding in our study was the moderating influence of energy levels on 30-day morality postsurgical treatment. Patients with higher ICH energy levels may benefit more from surgical intervention, which likely stems from an increased risk of ICH expansion [16]. Consequently, early surgical intervention and the application of hemostatic materials could be crucial in prevention of further rebleeding. Interestingly, another study with comparable number of patients found that in both high and low energy groups, well-known outcome predictors like GCS score, glucose level, ICH volume, or presence of IVH [10] were significantly correlated with mortality only in patients exhibiting high ICH energy. This finding might indicate a divergent natural progression of the disease between these two patient groups. Therefore, further research into radiomics in ICH management could aid in clarifying the role of surgical treatment, including the identification of patient subgroups which might distinctly benefit from it.
Our research also identified a significant moderating role of volume in the connection between 30-day mortality and surgical intervention. Following an ICH removal, a significant reduction in 30-day mortality was observed only in patients with larger ICH volumes. As previously documented in the literature, ICH size is one of the most important predictors of outcome [17]. Larger ICHs are associated with a mass effect, potentially leading to brain herniation. Additionally, a greater volume of blood can amplify its toxic impact on brain tissue [18] and is linked to an increased risk of ICH growth and early deterioration [19]. Our findings are consistent with other researchers who have also proposed that larger ICHs are an indication for surgical intervention [4, 5]. Other research groups also found that patients with such ICHs are more frequently candidates for surgery [20]. In contrast to ICH energy, common risk factors had similar impacts on mortality both in groups with larger and smaller ICH.
Moreover, we identified a moderating negative impact of IVH on mortality after surgical treatment. This is consistent with previous studies, indicating that patients with IVH did not benefit from surgical interventions [6, 21]. Presence of IVH has been previously established as independent factor for increased mortality after ICH [22]. Furthermore, it has been observed that patients with IVH are less likely to undergo surgery [20].
Our study highlights the value of radiomics analysis in aiding clinical decision-making for the treatment of patients with spontaneous ICH. We found that patients with higher volume and energy of ICH might benefit from surgical treatment, although its effectiveness could be diminished in cases with intraventricular blood; however, our study encountered certain limitations. Firstly, it was nonrandomized, and due to the absence of definitive guidelines for surgical treatment, decisions were based on clinical experience, potentially introducing bias. Another limitation is its single-center, retrospective nature. Hence, a larger multicenter randomized control trial is warranted to establish comprehensive ICH management guidelines that incorporate radiomic analysis. Despite these constraints, our study is pioneering in demonstrating the role of radiomics in influencing the outcomes of surgical treatment.
References
Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245.
Pinho J, Costa AS, Araújo JM, Amorim JM, Ferreira C. Intracerebral hemorrhage outcome: A comprehensive update. J Neurol Sci. 2019;398:54–66.
van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–76.
Veltkamp R, Purrucker J. Management of Spontaneous Intracerebral Hemorrhage. Curr Neurol Neurosci Rep. 2017;17.
Gregson BA, Broderick JP, Auer LM, et al. Individual patient data subgroup meta-analysis of surgery for Spontaneous Supratentorial Intracerebral Haemorrhage. Stroke. 2012;43:1496.
Mendelow AD, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005;365:387–97.
Vidale S, Bellocchi S, Taborelli A. Surgery for cerebral haemor-rhage—STICH II trial. Lancet. 2013;382:1401–2.
Fiorella D, Arthur AS, Mocco JD. 305 The INVEST Trial. Neurosurgery. 63:187. 2016.
Hanley DF, Thompson RE, Muschelli J, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15:1228–37.
Greenberg SM, Ziai WC, Cordonnier C, et al. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53:282–361.
Haider SP, Qureshi AI, Jain A, et al. Admission computed tomography radiomic signatures outperform hematoma volume in predicting baseline clinical severity and functional outcome in the ATACH-2 trial intracerebral hemorrhage population. Eur J Neurol. 2021;28:2989–3000.
Chen Q, Zhu D, Liu J, et al. Clinical-radiomics Nomogram for Risk Estimation of Early Hematoma Expansion after Acute Intracerebral Hemorrhage. Acad Radiol. 2021;28:307–17.
Zhan C, Chen Q, Zhang M, et al. Radiomics for intracerebral hemorrhage: are all small hematomas benign? Br J Radiol. 2021;94.
Gadelmawla ES. A vision system for surface roughness characterization using the gray level co-occurrence matrix. NDT E Int. 2004;37:577–88.
Kliś KM, Krzyżewski RM, Kwinta BM, Stachura K, Gąsowski J. Computer-Assisted Analysis of Intracerebral Hemorrhage Shape and Density. World Neurosurg. 2018;120:e863–9.
Shen Q, Shan Y, Hu Z, et al. Quantitative parameters of CT texture analysis as potential markersfor early prediction of spontaneous intracranial hemorrhage enlargement. Eur Radiol. 2018;28:4389–96.
Panchal HN, Shah MS, Shah DS. Intracerebral Hemorrhage Score and Volume as an Independent Predictor of Mortality in Primary Intracerebral Hemorrhage Patients. Indian J Surg. 2015;77:302.
De Oliveira MAL. Surgery for spontaneous intracerebral hemorrhage. Crit Care. 2020;24.
Lv XN, Li Q. Imaging predictors for hematoma expansion in patients with intracerebral hemorrhage: A current review. Brain Hemorrhages. 2020;1:133–9.
Chen CJ, Ding D, Ironside N, et al. Predictors of Surgical Intervention in Patients with Spontaneous Intracerebral Hemorrhage. World Neurosurg. 2019;123:e700.
Wang WZ, Jiang B, Liu HM, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: results from a randomized clinical trial in China. Int J Stroke. 2009;4:11–6.
Al-Mufti F, Thabet AM, Singh T, El-Ghanem M, Amuluru K, Gandhi CD. Clinical and Radiographic Predictors of Intracerebral Hemorrhage Outcome. Interv Neurol. 2018;7:118.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R.M. Krzyżewski, B.M. Kwinta, K. Stachura, T.J. Popiela, R. Pułyk, A. Słowik, J. Gąsowski and K.M. Kliś declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
62_2024_1406_MOESM1_ESM.docx
Supplemental Methods - details about image segmentation and paramters calculation; Supplementary Table - Additional comparison of patients treated surgically and conservatively.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krzyżewski, R.M., Kwinta, B.M., Stachura, K. et al. Association of Imaging-based Predictors with Outcome in Different Treatment Options for Intracerebral Hemorrhage. Clin Neuroradiol (2024). https://doi.org/10.1007/s00062-024-01406-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00062-024-01406-2