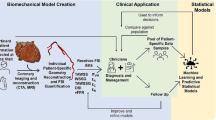
Abstract
Purpose
Assessing clot composition on prethrombectomy computed tomography (CT) imaging may help in stroke treatment planning. In this study we seek to use microCT imaging of fabricated blood clots to understand the relationship between CT radiographic signals and the biological makeup.
Methods
Clots (n = 10) retrieved by mechanical thrombectomy (MT) were collected, and 6 clot analogs of varying RBC composition were made. We performed paired microCT and histological image analysis of all 16 clots using a ScanCo microCT 100 (4.9 µm resolution) and standard H&E staining (imaged at 40×). From these data types, first order statistic (FOS) radiomics were computed from microCT, and percent composition of RBCs (%RBC) was computed from histology. Polynomial and linear regression (LR) were used to build statistical models based on retrieved thrombus microCT and %RBC that were evaluated for their ability to predict the %RBC of clot analogs from mean HU. Correlation analyses of microCT FOS with composition were completed for both retrieved clots and analogs.
Results
The LR model fits relating MT-retrieved clot %RBC with mean (R2 = 0.625, p = 0.006) and standard deviation (R2 = 0.564, p < 0.05) in HUs on microCT were significant. Similarly, LR models relating analog histological %RBC to analog protocol %RBC (R2 = 0.915, p = 0.003) and mean HUs on microCT (R2 = 0.872, p = 0.007) were also significant. When the LR model built using MT-retrieved clots was used to predict analog %RBC from mean HUs, significant correlation was observed between predictions and actual histological %RBC (R2 = 0.852, p = 0.009). For retrieved clots, significant correlations were observed for energy and total energy with %RBC and %FP (|R| > 0.7, q < 0.01). Analogs further demonstrated significant correlation between FOS energy, total energy, variance and %WBC (|R| > 0.9, q < 0.01).
Conclusion
MicroCT can be used to build models that predict AIS clot composition from routine CT parameters and help us to better understand radiomic signatures associated with clot composition and first pass outcomes. In future work, such observations can be used to better infer clot composition and inform thrombectomy prognostics from pretreatment CTs.




Similar content being viewed by others
References
Fiehler J, Gerloff C. Mechanical thrombectomy in stroke. Dtsch Arztebl Int. 2015;112(49):830.
Johnson S, McCarthy R, Gilvarry M, McHugh PE, McGarry JP. Investigating the mechanical behavior of clot analogues through experimental and computational analysis. Ann Biomed Eng. 2021;49:420–31.
Shin JW, Jeong HS, Kwon H‑J, Song KS, Kim J. High red blood cell composition in clots is associated with successful recanalization during intra-arterial thrombectomy. PLoS ONE. 2018;13(5):e197492.
Singh P, Kaur R, Kaur A. Clot composition and treatment approach to acute ischemic stroke: the road so far. Ann Indian Acad Neurol. 2013;16(4):494.
Xu R‑G, Ariëns RA. Insights into the composition of stroke thrombi: heterogeneity and distinct clot areas impact treatment. Haematologica. 2020;105(2):257.
Mousavi Janbeh Sarayi SM, et al. Vascular cross-section, rather than tortuosity, can classify first-pass outcome of mechanical thrombectomy for Ischemic stroke. Stroke Vasc Interv Neurol. 2023;3(2):e646.
Hernández-Fernández F, et al. Fibrin-platelet clots in acute ischemic stroke. Predictors and clinical significance in a mechanical thrombectomy series. Front Neurol. 2021;12:631343.
Maekawa K, et al. Erythrocyte-rich thrombus is associated with reduced number of maneuvers and procedure time in patients with acute ischemic stroke undergoing mechanical thrombectomy. Cerebrovasc Dis Extra. 2018;8(1):39–49.
LaGrange DD, et al. Predictive value of clot imaging in acute ischemic stroke: a systematic review of artificial intelligence and conventional studies. Neurosci Informatics. 2022; https://doi.org/10.1016/j.neuri.2022.100114.
Hanning U, et al. Imaging-based prediction of histological clot composition from admission CT imaging. J NeuroIntervent Surg. 2021;13(11):1053–7.
Wang C, et al. A nomogram for predicting thrombus composition in stroke patients with large vessel occlusion: combination of thrombus density and perviousness with clinical features. Neuroradiology. 2023;65(2):371–80.
Santo BA, et al. Multimodal CT imaging of ischemic stroke thrombi identifies scale-invariant radiomic features that reflect clot biology. J NeuroIntervent Surg. 2023; https://doi.org/10.1136/jnis-2022-019967.
Duffy S, et al. Novel methodology to replicate clot analogs with diverse composition in acute ischemic stroke. J NeuroIntervent Surg. 2017;9(5):486–91.
Fitzgerald S, et al. Novel human acute ischemic stroke blood clot analogs for in vitro thrombectomy testing. AJNR Am J Neuroradiol. 2021;42(7):1250–7.
Johnson S, Duffy S, Gunning G, Gilvarry M, McGarry J, McHugh P. Review of mechanical testing and modelling of thrombus material for vascular implant and device design. Ann Biomed Eng. 2017;45:2494–508.
Mousavi SJ, et al. Realistic computer modelling of stent retriever thrombectomy: a hybrid finite-element analysis-smoothed particle hydrodynamics model. J R Soc Interface. 2021;18(185):20210583.
Tutino VM, et al. Circulating neutrophil transcriptome may reveal intracranial aneurysm signature. PLoS ONE. 2018;13(1):e191407.
Shinohara RT, et al. Statistical normalization techniques for magnetic resonance imaging. Neuroimage Clin. 2014;6:9–19.
Lie W‑N. Automatic target segmentation by locally adaptive image thresholding. IEEE Trans Image Process. 1995;4(7):1036–41.
Aerts HJ, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun. 2014;5(1):1–9.
Thissen D, Steinberg L, Kuang D. Quick and easy implementation of the Benjamini-Hochberg procedure for controlling the false positive rate in multiple comparisons. J Educ Behav Stat. 2002;27(1):77–83.
Nelson LS. The Anderson-Darling test for normality. J Qual Technol. 1998;30(3):298.
Hedna VS, et al. Hemispheric differences in ischemic stroke: is left-hemisphere stroke more common? J Clin Neurol. 2013;9(2):97–102.
Dumitriu LaGrange D, et al. MicroCT can characterize clots retrieved with mechanical thrombectomy from acute ischemic stroke patients—a preliminary report. Front Neurol. 2022;13:824091.
Patel TR, et al. Histomic-based clot structure quantification for prediction of ischemic stroke etiology. Circulation. 2022;146(1):A15640–A15640.
Patel TR, Santo B, Monteiro A, Waqas M, Siddiqui AH, Tutino V. Data-driven ischemic stroke clot phenotyping from whole-slide histopathology images. 2021 IEEE Western New York Image and Signal Processing Workshop (WNYISPW). IEEE; 2021. pp. 1–5.
Patel TR, et al. Histologically interpretable clot radiomic features predict treatment outcomes of mechanical thrombectomy for ischemic stroke. Neuroradiology. 2023; https://doi.org/10.1007/s00234-022-03109-2.
Patel TR, et al. Biologically informed clot histomics are predictive of acute ischemic stroke etiology. Stroke Vasc Interv Neurol. 2023;3(2):e536.
Liu Y, et al. Quantification of clot spatial heterogeneity and its impact on thrombectomy. J NeuroIntervent Surg. 2021; https://doi.org/10.1136/neurintsurg-2021-018075.
Patel T, et al. Increased perviousness on CT for acute ischemic stroke is associated with fibrin/platelet-rich clots. AJNR Am J Neuroradiol. 2021;42(1):57–64.
Benson JC, et al. Clot permeability and histopathology: is a clot’s perviousness on CT imaging correlated with its histologic composition? J NeuroIntervent Surg. 2020;12(1):38–42.
Minnerup J, Kleinschnitz C. Visualization of clot composition in ischemic stroke: do we get what we see? Am Heart Assoc. 2011;42:1193–4.
Fitzgerald S, et al. Per-pass analysis of acute ischemic stroke clots: impact of stroke etiology on extracted clot area and histological composition. J NeuroIntervent Surg. 2021;13(12):1111–6.
Henninger N, Bouley J, Bråtane BT, Bastan B, Shea M, Fisher M. Laser Doppler flowmetry predicts occlusion but not tPA-mediated reperfusion success after rat embolic stroke. Exp Neurol. 2009;215(2):290–7.
Henninger N, Sicard KM, Schmidt KF, Bardutzky J, Fisher M. Comparison of ischemic lesion evolution in embolic versus mechanical middle cerebral artery occlusion in Sprague Dawley rats using diffusion and perfusion imaging. Stroke. 2006;37(5):1283–7.
Tashiro K, Shobayashi Y, Hotta A. Numerical simulation of non-linear loading-unloading hysteresis behavior of blood clots. Biocybern Biomed Eng. 2022;42(4):1205–17.
Acknowledgements
The authors would like to acknowledge Jay Shah for assistance in clot fabrication. Histology data in this study was generated with the assistance of the Histology Core Laboratory at the University at Buffalo’s Jacobs School of Medicine and Biomedical Sciences. MicroCT data were acquired at the University of Buffalo’s Optical Imaging and Analysis Facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
B.A. Santo, T.D. Jenkins, S.-S.K. Ciecierska and A.A. Baig declare that they have no competing interests. E.I. Levy—Board Membership: Stryker, NeXtGen Biologics, MedX Health, Cognition Medical, EndoStream; Consultancy: Claret Medical, GLG Consulting, Guidepoint, Imperative Care, Medtronic, Rebound Therapeutics, StimMed; Employment: University at Buffalo Neurosurgery Inc; Expert Testimony: renders medical/legal opinions as an expert witness; Stock/Stock Options: NeXtGen Biologics, Cognition Medical, Rapid Medical, Claret Medical, Imperative Care, Rebound Therapeutics, StimMed. A.H. Siddiqui—Financial interest/investor/stock options/ownership: Adona Medical, Inc., Amnis Therapeutics, BlinkTBI, Inc., Boston Scientific Corp. (for purchase of Claret Medical), Buffalo Technology Partners, Inc., Cardinal Consultants, LLC, Cerebrotech Medical Systems, Inc., Cognition Medical, Endostream Medical, Ltd, Imperative Care, Inc., International Medical Distribution Partners, Neurovascular Diagnostics, Inc., Q’Apel Medical, Inc., Radical Catheter Technologies, Inc., Rebound Therapeutics Corp. (purchased 2019 by Integra Lifesciences, Corp.), Rist Neurovascular, Inc., Sense Diagnostics, Inc., Serenity Medical, Inc., Silk Road Medical, Spinnaker Medical, Inc., StimMed, Synchron, Three Rivers Medical, Inc., Vastrax, LLC, VICIS, Inc., Viseon, Inc.; consultant/advisory board: Amnis Therapeutics, Boston Scientific, Canon Medical Systems USA, Inc., Cerebrotech Medical Systems, Inc., Cerenovus, Corindus, Inc., Endostream Medical, Ltd, Imperative Care, Inc., Integra LifeSciences Corp., Medtronic, MicroVention, Minnetronix Neuro, Inc., Northwest University—DSMB Chair for HEAT Trial, Penumbra, Q’Apel Medical, Inc., Rapid Medical, Rebound Therapeutics Corp., Serenity Medical, Inc., Silk Road Medical, StimMed, Stryker, Three Rivers Medical, Inc., VasSol, W.L. Gore & Associates; national PI/steering committees: Cerenovus LARGE trial and ARISE II trial, Medtronic SWIFT PRIME and SWIFT DIRECT trials, MicroVention FRED Trial and CONFIDENCE study, MUSC POSITIVE trial, Penumbra 3D Separator trial, COMPASS trial, INVEST trial; research grants: co-investigator, NIH/NINDS 1R01NS091075 Virtual Intervention of Intracranial Aneurysms; role: co-principal investigator, NIH-NINDS R21 NS109575-01 Optimizing Approaches to Endovascular Therapy of Acute Ischemic Stroke. V.M. Tutino—Financial interest/investor/stock options/ownership: Neurovascular Diagnostics, Inc., QAS.AI, Inc.; Consultant/advisory board: Canon Medical Systems USA; Research grants: Principal investigator, National Science Foundation Award No. 1746694 and NIH NINDS award R43 NS115314‑0; awardee of a Brain Aneurysm Foundation grant, a Center for Advanced Technology grant, and a Cummings Foundation grant.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors Briana A. Santo and TaJania D. Jenkins contributed equally to the manuscript.
Supplementary Information
62_2023_1380_MOESM1_ESM.pdf
Supplemental Table 1: Percent Compositions of All Retrieved Clots. Supplemental Table 2: Summary of radiomic feature and histological composition correlations for retrieved clots and synthetic clot analogs. Supplemental Figure 1: Iodine-stained and unstained thrombi were qualitatively and quantitatively comparable by histology. Supplemental Figure 2: Summary of thrombectomy outcome and clot image data for the patient cohort. Supplemental Figure 3: Clot analog histological compositions reflected RBC proportions used in the experimental protocol
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santo, B.A., Jenkins, T.D., Ciecierska, SS.K. et al. MicroCT and Histological Analysis of Clot Composition in Acute Ischemic Stroke. Clin Neuroradiol (2024). https://doi.org/10.1007/s00062-023-01380-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00062-023-01380-1