Abstract
Purpose
The most common protocols in the initial diagnostic of acute ischemic stroke do not assess cardiogenic or aortic causes of embolism. These are usually evaluated later by transthoracic (TTE) or transesophageal (TEE) echocardiography. This study aimed to evaluate the feasibility of a diagnostic tool for thoracic cardiovascular thrombi according to the first experience with a new extended cardio-stroke protocol (Big 5—Jena eCS protocol) in acute stroke patients.
Methods
Retrospective analyses of the tomography scans database of the Jena University Hospital were performed. We included a total of 67 patients in the feasibility analyses, based on the evaluation of three outcomes.
Results
Primary outcome: the Big 5—Jena eCS protocol was able to detect thoracic cardiovascular thrombi in a total of 20 patients in different locations including the arch of the aorta, the aortic valve, the left atrium, the left atrial appendage, the left ventricle, and the pulmonary arteries. Secondary outcome: implementating the protocol did not result in a significant elevation of the radiation exposure compared to traditional protocols. Tertiary outcome: the new protocol identified seven cases that were considered negative by echocardiography.
Conclusion
The implementation of an extended cardio-stroke protocol is feasible, no significantly time-consuming, acquiring assessable imaging, and maintaining radiation exposure acceptable. The Big 5—Jena eCS protocol was also able to detect some thrombi not reported by TTE or TEE; however, due to our data’s explorative character, a conclusive comparison with cardiac ultrasound is not possible. A prospective pilot study and clinical trials should be conducted to assess the diagnostic accuracy of this protocol compared to echocardiography and determine the potential impact on diagnostic and treatment decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is one of the most common diagnoses seen in the emergency department and contributes to significant morbidity and mortality in the adult population [1,2,3,4]. A substantial number of stroke patients are often affected by underlying heart conditions leading to an ischemic event [4, 5]. About 80–85% of strokes are ischemic events caused by a cerebral artery’s acute occlusion [6,7,8]. Based on the TOAST criteria of suspected etiology (Trial of ORG 10172 in Acute Stroke Treatment), ischemic strokes can be classified as cardioembolic, large artery atherosclerosis, small vessel disease, other known etiology, or undetermined etiologies [5, 9]. Cardiogenic embolism is responsible for about 20–40% of large vessel occlusion in stroke patients [2, 7, 10, 11].
The examination of the brain through a non-contrast brain computed tomography (CT), perfusion tomography (CT perfusion) of the brain, and CT angiography (CTA) of the cerebral arteries are commonly used protocols in the diagnosis of an acute ischemic stroke [12, 13]. Typical localization sites of cardiac thrombi such as the left atrium (LA), the left atrial appendage (LAA), or the aortic valve (AV) are not detected in those examinations [5, 14]. Transthoracic echocardiography (TTE) as a non-invasive method and transesophageal echocardiography (TEE) as a semi-invasive examination are the clinical standard for detection of cardiac thrombi [10, 11, 15]; however, the implementation of these examinations requires a clinically stable patient and is time-consuming. Therefore, these examination techniques are not indicated in the context of an acute stroke.
Cardiac CT might be a reliable alternative, even in the context of acute ischemic stroke in the emergency department (ED) [16,17,18]. By avoiding performing traditional (semi) invasive examinations for diagnosing cardiac thrombi, the complications associated with these methods can also be abolished. Furthermore, the additional information about thrombotic findings in the heart and aorta as soon as the patient arrives in the ED could potentially have an impact in early stroke treatment [1, 3, 10]. Therefore, the present study aimed to evaluate the feasibility of a diagnostic tool for cardiogenic and vascular thrombi based on the experience with a new extended cardio-stroke protocol (Big 5—Jena eCS protocol) in acute stroke patients. Thus, the study ambition was not related to prove diagnostic accuracy or to test processes that future clinical trials could use in order to prove diagnostic accuracy (the methodology of a pilot study). In the present study the main question was to evaluate whether a radiological test works. In this scenario, the most recent literature suggests that feasibility studies are the appropriate methodological design [19].
Material and Methods
Big 5—Jena eCS Protocol Design
At the Institute for Diagnostic and Interventional Radiology (IDIR) of the Jena University Hospital (Universitätsklinikum Jena [UKJ]) in Thuringia, Germany, we designed an electrocardiogram (ECG) gated CT protocol combining diagnostics for acute ischemic stroke including brain perfusion, cerebral arteries, and thoracic cardiovascular thrombi (extended cardio-stroke protocol, Big 5—Jena eCS protocol).
This protocol is performed using a multi-slice (256 detector row) GE Revolution CT Scanner (General Electric Healthcare, Chicago, IL, USA), and 110 ml of contrast medium Iomeprol 400 mg iodine/ml (Imeron® 400 MCT Bracco Imaging, Milan, Italy). As a result, we can obtain the following in a single examination:
-
1.
A non-enhanced brain CT.
-
2.
A CTA involving a combination of an ECG-triggered axial scan over the heart and the arch of aorta followed by a helical scan without triggering up to the brain. Contrast medium (CM) application: 60 ml (4 ml/s).
-
3.
A venous axial scan of the LAA.
-
4.
A CT perfusion of the brain. CM application: 50 ml (4 ml/s).
Compared with the standard imaging in stroke patients, our new protocol increases the intravenous contrast to 110 ml, which means only a 10 ml addition.
The ECG triggering is made using a cardiac profile depending on the heart rate. The bolus tracking is performed by defining a threshold of 110 Hounsfield units (HU) in the ascending aorta with a delay of 6 s. The scan time for a 180° scan over the heart is 140ms. A schema of our new Big 5—Jena eCS protocol is presented in Fig. 1.
With this protocol, we can evaluate five essential aspects in the context of stroke. Firstly, we can assess the cerebral tissue, the cerebral perfusion, and the intracranial arteries responsible for the brain’s blood supply. We can also evaluate the arch of aorta (AA), the vertebral arteries (VA), and the carotid arteries. Furthermore, we also visualize the heart structures, including the left ventricle (LV), the aortic valve (AV), the LA, and the LAA.
Sample
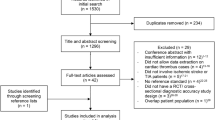
Overall, 1133 consecutive patients with acute stroke symptoms were admitted in the ED of the UKJ and 1053 patients were assessed with the new Big 5—Jena eCS protocol. This number was reached based on the exclusion of 80 patients who underwent a previous non-enhanced brain CT and CTA before being transferred to the ED of the UKJ. We included a subsample of patients that underwent endovascular thrombectomy to conduct the analyses, as these patients were more likely to have underlying thoracic cardiovascular thrombi relative to those on who no endovascular thrombectomy was performed. This procedure yielded a total of 67 participants after excluding 986 patients where no endovascular thrombectomy was performed (see Fig. 2).
Data Analysis
The research team reviewed first the scans obtained by implementing the Big 5—Jena eCS protocol and then proceeded to analyze the echocardiographic reports. In the case of the new protocol, three assessors, a second-year medical resident, and two experienced board-certified radiologists of our ND, one with vast experience in cardiovascular radiology and the other in neuroradiology, independently reviewed post hoc each of the 67 CT scans. Assessors were blinded to the echocardiography results, and any conflicts were discussed until the three assessors reached a consensus.
In the case of the echocardiographic data, examination was performed at a median of 9 days after hospital admission (range 7–30 days). Of the 67 patients 49 underwent echocardiography to identify a potential thrombotic source for the ischemic stroke. We did not perform a systematic secondary analysis and relied on the original data as available from non-contrast echocardiographic assessments of patients performed at the treating physicians’ discretion without systematic secondary analysis by a core laboratory. A TTE was performed in 22 patients and TEE in 27. Echocardiography was not performed in the remaining patients, in 1 case due to early transfer to another hospital, in 3 patients due to the prior evidence of cardiovascular thrombi in the CT scan, in 4 cases due to treating physician’s judgement and in 10 patients due to death after hospital admission.
Study Outcomes
Based on this information the research team evaluated three outcomes. The primary outcome evaluated the Big 5—Jena eCS protocol’s ability to detect thoracic cardiovascular thrombi. The secondary outcome evaluated the mean dose length product (DLP) as an index for radiation exposure and overall safety. The tertiary outcome devolved on the possibility of the protocol to detect thrombotic deposits not reported by echocardiography.
Results
A total of 67 patient were included in the feasibility analyses. The general characteristics of the sample of participants are shown in Table 1. There were 37 women (55.2%) and 30 men (44.8%), with a mean age of 70 years ± 11 years (range 46–87 years). Forty patients had atrial fibrillation (AF), and one patient had atrial flutter. Almost half of the patients with AF, i.e., 19 cases, were new-onset diagnoses.
Primary Outcome
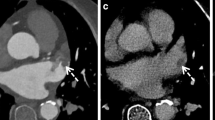
In CT scans performed by following the Big 5—Jena eCS protocol, thoracic cardiovascular thrombotic findings were observed in 20 patients (29.9%). The primary localization of those thrombi was the AV, which was affected in eight patients, followed by the AA in six individuals and the LAA in five. Furthermore, thrombi were also observed in the LA of four patients, the LV in two patients, and one patient was diagnosed with a pulmonary embolism. An overview of the blood vessel occlusions (carotids, vertebral arteries, and intracranial circulation) associated with the thrombi’s localization in the heart, the aorta, and the pulmonary arteries (PA), including the overlaps, is given in Table 2. Of the 41 patients with cardiac arrhythmias (40 with AF and one with atrial flutter, see Table 1), 13 patients (31.7%) showed thoracic cardiovascular thrombotic findings in CT as follows: two in the LAA, three in the LAA + LA, one in the LA, three on the AV, one on the AV + AA, two in the AA, and one in the LV + PA. For example, Fig. 3 shows the case of a patient admitted to the ED of UKJ in which the Big 5—Jena eCS protocol was implemented. There we can see that thrombi in the LAA and LA were identified, along with a right M1 occlusion.
Imaging of a 73-year-old female treated endovascularly at our hospital after initial admission with suspicion of a right hemispheric stroke, occlusion of the middle cerebral artery’s first segment, and simultaneously thrombi in the left atrium and left atrial appendage. a Non-enhanced brain computed tomography (CT) without any stroke signs, b CT angiography showing thrombosis of the right middle cerebral artery’s first segment (white arrow), c coronal, and d axial heart scans show a thrombus in the left atrium (black arrow) and left atrial appendage (white arrow). The perfusion parameters show e increased mean transit time and f time to peak with no significant alteration of g the cerebral blood flow and h the cerebral blood volume in the right middle cerebral artery’s perfusion territory
Secondary Outcome
The extended cardio-stroke protocol also leads to slightly higher radiation exposure. In our group of 20 patients with thoracic cardiovascular thrombotic findings, the mean dose length product (DLP) was 2072.5 mGy*cm with a standard deviation (SD) of 360.3 mGy*cm. In a group of 100 patients examined during the same period with a non-enhanced brain CT, CT perfusion, and CTA, the mean DLP was 2038.8 mGy*cm with an SD of 236.1 mGy*cm.
Tertiary Outcome
Of the 67 participants, the Big 5—Jena eCS protocol detected thoracic cardiovascular thrombi in 20 individuals. Among the 20 individuals in which the CT was positive, only 12 cardiac ultrasounds were performed. This was due to the fact that one patient was transferred to another hospital and two patients died before cardiac echocardiography could be performed; additionally, two patients did not undergo echocardiography based on the treating physician’s judgement and three patients due to the prior evidence of cardiovascular thrombi in the CT scan.
Of the 20 patients with CT-detected cardiovascular thrombotic findings, 4 underwent a TTE and 8 a TEE (40% of cases with suspected thrombi based on CT findings). The TTE was positive in two patients: one patient with thrombus in the LV and one in the LA and negative in two cases with CT-diagnosed thrombi: one with thrombus in the LAA and another with aggregations on the AV. The TEE was positive in three cases: one with thrombi in the LAA, one with thrombi in the LV and a pulmonary embolism, and another on the AV, in which TEE detected spontaneous echocardiographic contrast and negative in five patients: one with affectation of the AA, two with aggregations on the AV, and two patients with simultaneous affectation of the AV and AA. As expected, due to the methodological limitations, the compromise of the AA could not be diagnosed through TTE. Thus, there was no sufficient echocardiographic confirmation in cases with suspected thrombi in CT.
Discussion
There are already studies comparing echocardiography and cardiac CT for cardiovascular cause finding in stroke patients, but not in the context of acute stroke [1, 4, 8, 10, 11, 16, 18, 20]. We have developed a CT protocol for this purpose, and our initial experience with it has shown that such an approach is possible: the resulting imaging is of sufficient quality, acquisition times are not significantly longer than usual, the incrementation of radiation exposure is minimal, and the contrast medium application was slightly higher compared to the most common protocols. Finally, the protocol was also able to find some important radiological findings that echocardiography might have missed.
The non-systematically quantified time required to set up the ECG triggering was less than 5 min, which was acceptable; however, in recent months, to reduce direct contact with patients whose coronavirus disease 2019 (COVID-19) status is unknown, the ECG triggering was eliminated without significantly affecting image quality. At the same time, the cardiac window was widened, and currently, the entire lung field is being acquired to assess possible pulmonary infiltrates that could indicate a COVID-19 infection.
Regarding the DLP findings, technical advances regarding CT acquisition images, including the iterative reconstruction, have considerably reduced the radiation exposure [3, 4, 21]. The extended cardio-stroke protocol leads to slightly higher radiation exposure than the usual CT protocols [22,23,24,25]; however, due to the equivalence of CT and TEE described in the literature regarding thrombus detection in patients after a stroke [5, 15, 17] and the additional possibility of evaluation of the aortic arch, the Big 5—Jena eCS protocol has been used as clinical standard at our radiology institute in the initial assessment of patients with acute stroke and suspicion of an ischemic etiology.
Early phase CT images have great sensitivity in the detection of thrombi [26]. Still, its specificity is suboptimal because of its inability to characterize an apparent filling defect differentiating it between blood stasis and an actual thrombus, leading to easy misdiagnoses of LAA thrombi [1, 10, 27]. In an attempt to avoid this pitfall, we implemented in our study protocol two contrast phases: the initial CTA, which includes the LAA in an early arterial phase, and a late venous phase confined just to the LAA, which allows a homogeneous enhancement of this cavity [1, 24]. Compared to the echocardiography, the CT offers a superior anatomical visualization of the heart structures, allowing the evaluation of LAA and LA enlargement. Noteworthy, circulatory stasis, which is often detected before an LAA thrombus manifests, and LAA/LA enlargement are considered markers of embolism and predictors for stroke recurrence, even independent of atrial fibrillation [4, 28, 29].
Due to its safety and wide availability, the TEE is well implemented in the clinical routine for evaluating stroke patients [10, 30, 31]. Additionally, the TEE is a semi-invasive, time-consuming technique that requires specially trained and skilled healthcare staff to be accurately performed and interpreted [30,31,32]. Nevertheless, TEE is only sensitive enough to adequately evaluate the LAA, LA, and AV. On the other hand, the TTE is a non-invasive procedure and is not associated with the TEE complications, besides being more sensitive for thrombi in the LV; however, it should be noted that methodologically, it is impossible to evaluate the AA by TTE [28,29,30, 33].
This single center study is retrospective, descriptive, and therefore bounded by some limitations. A major one is the very explorative character of the data. Additionally, no medication was administered to decelerate the heart rate according to the acute stroke setting. Despite ECG gating, motion artifacts because of normal or even increased heart rate could not be wholly avoided. Therefore, rapid heart rate and motion artifacts can lead to misdiagnosis of small thrombi in the coronary arteries; however, this is not a primary aspect to assess with the Big 5—Jena eCS protocol. Another limitation regarding the echocardiographic report is their operator-dependent nature; thus, our echocardiographers’ experience might affect the results we obtained from the reports. As mentioned, echocardiography was performed days after hospital admission. As part of the medical and endovascular treatment, the thrombolytics administration might have dissolved blood clots when the cardiac ultrasound took place. The anatomical method-related limitations and the therapeutic effects might contribute to the so-called missing findings in the TTE and TEE.
Irrespective of the abovementioned limitations, the cardiac imaging included in our extended cardio-stroke protocol also allows the simultaneous evaluation of atherosclerotic disease as another embolism substrate [3, 4, 34, 35], which could have important therapeutic implications in embolic stroke associated with atrial fibrillation [36,37,38]. The non-invasive character of CT combined with its reproducibility and the other advantages mentioned above highlight this technique as a reliable option in the assessment of acute stroke patients in the ED [8, 25]. Furthermore, it is possible that the presence of an intracavitary thrombus could support the anticoagulation despite prior fibrinolysis for selected patients with a small or moderate sized brain infarction and no evidence of brain bleeding in the CT scan. Diagnosing the cardiac thrombus simultaneously with the stroke may support early anticoagulation in patients with a high risk for short-term recurrent stroke. A study in Lombardy (Italy) suggested that patients with atrial fibrillation treated with early anticoagulation after an acute stroke had a lower incidence of clinically symptomatic intracranial hemorrhage and that anticoagulation < 48 h was associated with fewer ischemic recurrences in selected patients, without increasing the risk of intracerebral bleeding [36, 39].
Conclusion
In our first experience implementing the new extended cardio-stroke protocol (Big 5—Jena eCS protocol) regularly, we could establish the protocol’s feasibility in the context of acute stroke. In addition, the new protocol allowed the rapid acquisition of adequate imaging without significantly incrementing the radiation exposure or the contrast medium administration.
Due to the explorative nature of the present feasibility study, we consider its value lies in the generation of a hypothesis that should be confirmed with more robust methodologies in the future. Even though we did not formally evaluate the methodological procedures of the present research in order to reach the status of a pilot study, we think it is of value to highlight some challenges that pilot studies or clinical trials might face when evaluating a radiological procedure similar to the Big 5—Jena eCS protocol. For example, it is recommended that every patient is evaluated with the same diagnosing imaging (computed tomography and echocardiography) and that the design of the study should strive to be prospective in nature, so that the diagnostic accuracy of the Big 5—Jena eCS protocol can be compared to cardiac ultrasound.
References
Ajlan AM, Bagdadi RR, Alama MN, Ayoub O. Impact of Implementing Cardiac CT in Evaluating Patients Suspected of Cardioembolic Stroke. J Comput Assist Tomogr. 2016;40:380–6.
Al-Farsi K, Siddiqui AA, Sharef YW, Al-Belushi AK, Al-Hashim H, Al-Ghailani M, Johnston WJ. Hemorrhagic cardioembolic stroke secondary to a left ventricular thrombus: a therapeutic dilemma. Oman Med J. 2013;28:56–9.
Hur J, Choi BW. Cardiac CT Imaging for Ischemic Stroke: Current and Evolving Clinical Applications. Radiology. 2017;283:14–28.
Hur J., Choi B.W. Ischemic Stroke: The Role of Cardiac CT. In: Schoepf U. (editor) CT of the Heart. Contemporary Medical Imaging. Humana, Totowa, NJ. 2019. pp. 635–645.
Camen S, Haeusler KG, Schnabel RB. Cardiac imaging after ischemic stroke: Echocardiography, CT, or MRI? Herz. 2019;44:296–303.
Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarría VV, Muñoz Venturelli P, Brunser A, Peng B, Cui L, Song L, Rogers K, Middleton S, Lim JY, Forshaw D, Lightbody CE, Woodward M, Pontes-Neto O, De Silva HA, Lin RT, Lee TH, Pandian JD, Mead GE, Robinson T, Watkins C; HeadPoST Investigators and Coordinators. Cluster-Randomized, Crossover Trial of Head Positioning in Acute Stroke. N Engl J Med. 2017;376:2437–47.
Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–40.
Boussel L, Cakmak S, Wintermark M, Nighoghossian N, Loffroy R, Coulon P, Derex L, Cho TH, Douek PC. Ischemic stroke: etiologic work-up with multidetector CT of heart and extra- and intracranial arteries. Radiology. 2011;258:206–12.
Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Jin KN, Chun EJ, Choi SI, Ko SM, Han MK, Bae HJ, Park JH. Cardioembolic origin in patients with embolic stroke: Spectrum of imaging findings on cardiac MDCT. AJR Am J Roentgenol. 2010;195:W38–44.
Choi YR, Kim HL, Kwon HM, Chun EJ, Ko SM, Yoo SM, Choi SI, Jin KN. Cardiac CT and MRI for Assessment of Cardioembolic Stroke. Cardiovasc Imaging Asia. 2017;1:13–22.
Diener HC, Weimar C, Berlit P, Deuschl G, Elger C, Gold R, Hacke W, Hufschmidt A, Mattle H, Meier U, Oertel WH, Reichmann H, Schmutzhard E, Wallesch CW, Weller M. Leitlinien für Diagnostik und Therapie in der Neurologie. Stuttgart, New York, Thieme, 2012.
Mayer TE, Hamann GF, Baranczyk J, Rosengarten B, Klotz E, Wiesmann M, Missler U, Schulte-Altedorneburg G, Brueckmann HJ. Dynamic CT perfusion imaging of acute stroke. AJNR Am J Neuroradiol. 2000;21:1441–9.
Haeusler KG, Gröschel K, Köhrmann M, Anker SD, Brachmann J, Böhm M, Diener HC, Doehner W, Endres M, Gerloff C, Huttner HB, Kaps M, Kirchhof P, Nabavi DG, Nolte CH, Pfeilschifter W, Pieske B, Poli S, Schäbitz WR, Thomalla G, Veltkamp R, Steiner T, Laufs U, Röther J, Wachter R, Schnabel R. Expert opinion paper on atrial fibrillation detection after ischemic stroke. Clin Res Cardiol. 2018;107:871–80.
Budoff MJ, Shittu A, Hacioglu Y, Gang E, Li D, Bhatia H, Alvergue J, Karlsberg RP. Comparison of transesophageal echocardiography versus computed tomography for detection of left atrial appendage filling defect (thrombus). Am J Cardiol. 2014;113:173–7.
Pagán RJ, Parikh PP, Mergo PJ, Gerber TC, Mankad R, Freeman WD, Shapiro BP. Emerging role of cardiovascular CT and MRI in the evaluation of stroke. AJR Am J Roentgenol. 2015;204:269–80.
Sipola P, Hedman M, Onatsu J, Turpeinen A, Halinen M, Jäkälä P, Vanninen R. Computed tomography and echocardiography together reveal more high-risk findings than echocardiography alone in the diagnostics of stroke etiology. Cerebrovasc Dis. 2013;35:521–30.
Hur J, Kim YJ, Lee HJ, Ha JW, Heo JH, Choi EY, Shim CY, Kim TH, Nam JE, Choe KO, Choi BW. Cardiac computed tomographic angiography for detection of cardiac sources of embolism in stroke patients. Stroke. 2009;40:2073–8.
Kenis SF, Abeyakoon O, Plumb AAO, Halligan S. Do radiological research articles apply the term “pilot study” correctly? Systematic review. Clin Radiol. 2020;75:395.e1–5.
Hur J, Kim YJ, Lee HJ, Nam JE, Ha JW, Heo JH, Chang HJ, Kim HS, Hong YJ, Kim HY, Choe KO, Choi BW. Dual-enhanced cardiac CT for detection of left atrial appendage thrombus in patients with stroke: a prospective comparison study with transesophageal echocardiography. Stroke. 2011;42:2471–7.
Budoff MJ. Maximizing dose reductions with cardiac CT. Int J Cardiovasc Imaging. 2009;25:279–87.
Hausleiter J, Meyer T, Hermann F, Hadamitzky M, Krebs M, Gerber TC, McCollough C, Martinoff S, Kastrati A, Schömig A, Achenbach S. Estimated radiation dose associated with cardiac CT angiography. JAMA. 2009;301:500–7.
Hur J, Kim YJ, Nam JE, Choe KO, Choi EY, Shim CY, Choi BW. Thrombus in the left atrial appendage in stroke patients: detection with cardiac CT angiography – a preliminary report. Radiology. 2008;249:81–7.
Kim SC, Chun EJ, Choi SI, Lee SJ, Chang HJ, Han MK, Bae HJ, Park JH. Differentiation between spontaneous echocardiographic contrast and left atrial appendage thrombus in patients with suspected embolic stroke using two-phase multidetector computed tomography. Am J Cardiol. 2010;106:1174-81.
Furtado AD, Adraktas DD, Brasic N, Cheng SC, Ordovas K, Smith WS, Lewin MR, Chun K, Chien JD, Schaeffer S, Wintermark M. The triple rule-out for acute ischemic stroke: imaging the brain, carotid arteries, aorta, and heart. AJNR Am J Neuroradiol. 2010;31:1290–6.
Romero J, Husain SA, Kelesidis I, Sanz J, Medina HM, Garcia MJ. Detection of left atrial appendage thrombus by cardiac computed tomography in patients with atrial fibrillation: a meta-analysis. Circ Cardiovasc Imaging. 2013;6:185–94.
Zou H, Zhang Y, Tong J, Liu Z. Multidetector computed tomography for detecting left atrial/left atrial appendage thrombus: a meta-analysis. Intern Med J. 2015;45:1044–53.
Pathan F, Hecht H, Narula J, Marwick TH. Roles of Transesophageal Echocardiography and Cardiac Computed Tomography for Evaluation of Left Atrial Thrombus and Associated Pathology: A Review and Critical Analysis. JACC Cardiovasc Imaging. 2018;11:616–27.
Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23:961–9.
Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, Habib G, Ringelstein EB, Sicari R, Zamorano JL, Sitges M, Caso P; European Association of Echocardiography. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2010;11:461–76.
Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D; American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–76.
Ayres NA, Miller-Hance W, Fyfe DA, Stevenson JG, Sahn DJ, Young LT, Minich LL, Kimball TR, Geva T, Smith FC, Rychik J; Pediatric Council of the American Society of the Echocardiography. Indications and guidelines for performance of transesophageal echocardiography in the patient with pediatric acquired or congenital heart disease: report from the task force of the Pediatric Council of the American Society of Echocardiography. J Am Soc Echocardiogr. 2005;18:91–8.
Hilberath JN, Oakes DA, Shernan SK, Bulwer BE, D’Ambra MN, Eltzschig HK. Safety of transesophageal echocardiography. J Am Soc Echocardiogr. 2010;23:1115–27; quiz 1220–1.
Benjamin EJ, Plehn JF, D’Agostino RB, Belanger AJ, Comai K, Fuller DL, Wolf PA, Levy D. Mitral annular calcification and the risk of stroke in an elderly cohort. N Engl J Med. 1992;327:374–9.
Higgins J, Mayo J, Skarsgard P. Cardiac computed tomography facilitates operative planning in patients with mitral calcification. Ann Thorac Surg. 2013;95:e9–11.
Seiffge DJ, Werring DJ, Paciaroni M, Dawson J, Warach S, Milling TJ, Engelter ST, Fischer U, Norrving B. Timing of anticoagulation after recent ischaemic stroke in patients with atrial fibrillation. Lancet Neurol. 2019;18:117–26.
Tsivgoulis G, Safouris A, Kim DE, Alexandrov AV. Recent Advances in Primary and Secondary Prevention of Atherosclerotic Stroke. J Stroke. 2018;20:145–66. Erratum in: J Stroke. 2018;20:417.
Yaghi S, Mistry E, Liberman AL, Giles J, Asad SD, Liu A, Nagy M, Kaushal A, Azher I, Mac Grory B, Fakhri H, Brown Espaillat K, Pasupuleti H, Martin H, Tan J, Veerasamy M, Esenwa C, Cheng N, Moncrieffe K, Moeini-Naghani I, Siddu M, Scher E, Trivedi T, Lord A, Furie K, Keyrouz S, Nouh A, Leon Guerrero CR, de Havenon A, Khan M, Henninger N. Anticoagulation Type and Early Recurrence in Cardioembolic Stroke: The IAC Study. Stroke. 2020;51:2724–32.
Canavero I, Cavallini A, Sacchi L, Quaglini S, Arnò N, Perrone P, DeLodovici ML, Marcheselli S, Micieli G. Safely Addressing Patients with Atrial Fibrillation to Early Anticoagulation after Acute Stroke. J Stroke Cerebrovasc Dis. 2017;26:7–18.
Funding
This research received no funding.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
The first draft of the manuscript was written by Moisés Felipe Molina Fuentes, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
M.F. Molina-Fuentes, R. Neumann, W. Behringer, M. Franz, P. C. Schulze, O.W. Witte, A. Günther, C. Klingner, L. Lehmkuhl, B. Steiniger, U. Teichgräber, J.E. Rod and T.E. Mayer declare that they have no competing interests.
Ethical standards
For this article no studies with human participants or animals were performed by any of the authors. The protocol of this study was approved by the Friedrich Schiller University ethics committee and performed in accordance with the Declaration of Helsinki.
Additional information
Consent for publication: Not applicable.
Availability of Data and Material
Availability of data: The data is stored on the servers of the Jena University Hospital.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Molina-Fuentes, M.F., Neumann, R., Behringer, W. et al. Feasibility of the Big 5—Jena eCS Protocol. Clin Neuroradiol 31, 901–909 (2021). https://doi.org/10.1007/s00062-021-01058-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-021-01058-6