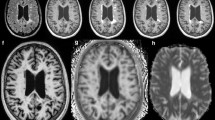
Abstract
Purpose
To evaluate the performance of an innovative image processing approach for detection of T2-weighted hyperintense multiple sclerosis (MS) lesions.
Methods
In this study 20 consecutive patients with inflammatory demyelinating lesions were retrospectively evaluated of whom 10 patients featured progressive disease and 10 a stable lesion load. 3 mm transversal FLAIRfusion imaging was processed and archived. Image processing was performed through landmark-based 3D co-registration of the previous and current isotropic FLAIR examination followed by inversion of image contrast. Thereby, the hyperintense signals of the unchanged MS plaques extinguish each other, while newly developed lesions appear bright on FLAIRfusion. Diagnostic performance was evaluated by 4 experienced readers. Consensus reading supplied the reference standard. Sensitivity, specificity, NPV (negative predictive value), PPV (positive predictive value), interreader agreement and reading time were the outcome measures analyzed.
Results
Combined sensitivity was 100% at a specificity of 88.2%, with PPV ranging from 83.3% to 90.1% and NPV at 100%. Reading time was nearly 5‑fold faster than conventional side by side comparison (35.6 s vs. 163.7 s, p < 0.001). Cohen’s kappa was excellent (>0.75; p < 0.001) and Cronbach’s alpha was 0.994.
Conclusion
FLAIRfusion provides reliable detection of newly developed MS lesions along with strong interreader agreement across all levels of expertise in 35 s of reading time.






Similar content being viewed by others
References
Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–17.
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for Multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302.
Comi G. Disease-modifying treatments for progressive multiple sclerosis. Mult Scler J. 2013;19:1428–36.
Río J1, Rovira A, Tintoré M, Huerga E, Nos C, Tellez N, Tur C, Comabella M, Montalban X. Relationship between MRI lesion activity and response to IFN-beta in relapsing-remitting Multiple sclerosis patients. Mult Scler J. 2008;14:479–84.
Prosperini L, Mancinelli CR, De Giglio L, De Angelis F, Barletta V, Pozzilli C. Interferon beta failure predicted by EMA criteria or isolated MRI activity in Multiple sclerosis. Mult Scler J. 2014;20:566–76.
Sormani MP, Bruzzi P. MRI lesions as a surrogate for relapses in Multiple sclerosis: a meta-analysis of randomised trials. Lancet Neurol. 2013;12:669–76.
Abraham AG, Duncan DD, Gange SJ, West S. Computer-aided assessment of diagnostic images for epidemiological research. BMC Med Res Methodol. 2009;9:74.
Dankerl P, Cavallaro A, Dietzel M, Tsymbal A, Kramer M, Seifert S, Uder M, Hammon M. Clinical evaluation of semi-automatic landmark-based lesion tracking software for CT-scans. Cancer Imaging. 2014;14:6.
Garcia-Lorenzo D, Francis S, Narayanan S, Arnold DL, Collins DL. Review of automatic segmentation methods of Multiple sclerosis white matter lesions on conventional magnetic resonance imaging. Med Image Anal. 2013;17:1–18.
Cabezas M, Oliver A, Roura E, Tsymbal A, Kramer M, Seifert S, Uder M, Hammon M. Automatic Multiple sclerosis lesion detection in brain MRI by FLAIR thresholding. Comput Methods Programs Biomed. 2014;115:147–61.
Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Mühlau M. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple sclerosis. Neuroimage. 2012;59:3774–83.
van Heerden J, Rawlinson D, Zhang AM, Chakravorty R, Tacey MA, Desmond PM, Gaillard F. Improving Multiple sclerosis plaque detection using a semiautomated assistive approach. AJNR Am J Neuroradiol. 2015;36:1465–71.
Moraal B, Meier DS, Poppe PA, Geurts JJ, Vrenken H, Jonker WM, Knol DL, van Schijndel RA, Pouwels PJ, Pohl C, Bauer L, Sandbrink R, Guttmann CR, Barkhof F. Subtraction MR images in a multiple sclerosis multicenter clinical trial setting. Radiology. 2009;250:506–14.
N’gbo N’gbo Ikazabo R, Mostosi C, Quivron B, Delberghe X, El Hafsi K, Lysandropoulos AP. Immune-reconstitution inflammatory syndrome in Multiple sclerosis patients treated with natalizumab: a series of 4 cases. Clin Ther. 2016;38:670–5.
Hodel J, Outteryck O, Dubron C, Dutouquet B, Benadjaoud MA, Duhin E, Verclytte S, Zins M, Luciani A, Rahmouni A, Pruvo JP, Vermersch P, Leclerc X. Asymptomatic progressive Multifocal Leukoencephalopathy associated with natalizumab: diagnostic precision with MR imaging. Radiology. 2016;278:863–72.
Dubey D, Cano CA, Stüve O. Update on monitoring and adverse effects of approved second-generation disease-modifying therapies in relapsing forms of Multiple sclerosis. Curr Opin Neurol. 2016;29(3):278-85.
Yousry TA, Pelletier D, Cadavid D, Gass A, Richert ND, Radue EW, Filippi M. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol. 2012;72:779–87.
Boster AL, Nicholas JA, Topalli I, Kisanuki YY, Pei W, Morgan-Followell B, Kirsch CF, Racke MK, Pitt D. Lessons learned from fatal progressive Multifocal Leukoencephalopathy in a patient with Multiple sclerosis treated with natalizumab. JAMA Neurol. 2013;70:398–402.
Traboulsee A, Simon JH, Stone L, Fisher E, Jones DE, Malhotra A, Newsome SD, Oh J, Reich DS, Richert N, Rammohan K, Khan O, Radue EW, Ford C, Halper J, Li D. Revised recommendations of the Consortium of MS Centers Task Force for a Standardized MRI Protocol and Clinical Guidelines for the Diagnosis and Follow-Up of Multiple Sclerosis. AJNR Am J Neuroradiol. 2016;37:394–401.
Clifford DB, De Luca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive Multifocal Leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9:438–46.
Honce JM, Nagae L, Nyberg E. Neuroimaging of natalizumab complications in Multiple sclerosis: PML and other associated entities. Mult Scler Int. 2015;2015:809252.
Rogosnitzky M, Branch S. Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals. 2016;29:365–76.
Runge VM. Safety of the gadolinium-based contrast agents for magnetic resonance imaging, focusing in part on their accumulation in the brain and especially the dentate nucleus. Invest Radiol. 2016;51:273–9.
Funding
This work was supported by the Interdisciplinary Center for Clinical Research (IZKF Erlangen, project J53, MAS). RAL holds an endowed professorship supported by NovartisPharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M. A. Schmidt, R. A. Linker, S. Lang, H. Lücking, T. Engelhorn, S. Kloska, M. Uder, A. Cavallaro, A. Dörfler and P. Dankerl declare that they have no competing interests.
Ethical standards
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Schmidt, M.A., Linker, R.A., Lang, S. et al. FLAIRfusion Processing with Contrast Inversion. Clin Neuroradiol 28, 367–376 (2018). https://doi.org/10.1007/s00062-017-0567-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00062-017-0567-y