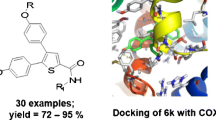
Abstract
Cyclooxygenase (COX), which plays a role in converting arachidonic acid to inflammatory mediators, could be inhibited by non-steroidal anti-inflammatory drugs (NSAIDs). Although potent NSAIDs are available for the treatment of pain, fever, and inflammation, some side effects, such as gastrointestinal ulcers, limit the use of these medications. In recent years, selective COX-2 inhibitors with a lower incidence of adverse effects attained an important position in medicinal chemistry. In order to introduce some new potent COX-2 inhibitors, a new series of 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amines was designed, synthesized, and evaluated. The docking studies performed by AutoDock Vina demonstrated that docked molecules were positioned as well as a crystallographic ligand in the COX-2 active site, and SO2Me pharmacophore was inserted into the secondary pocket of COX-2 and formed hydrogen bonds with the active site. The designed compounds were synthesized through two-step reactions. In the first step, different 1-(4-(methylsulfonyl)phenyl)-2-(phenylamino)ethan-1-one derivatives were obtained by the reaction of aniline derivatives and α-bromo-4-(methylsulfonyl)acetophenone. Then, condensation of intermediates with different 2-aminopyridines gave final compounds. Enzyme inhibition assay and formalin test were performed to evaluate the activity of these compounds. Among these compounds, 8-methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5n) exhibited the highest potency (IC50 = 0.07 µM) and selectivity (selectivity index = 508.6) against COX-2 enzyme (selectivity index: COX-1 IC50/COX-2 IC50). The antinociceptive activity assessment via the formalin test showed that nine derivatives (5a, 5d, 5h, 5i, 5k, 5q, 5r, 5s, and 5t) possessed significant activity compared with the control group with a p value less than 0.05.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachidonic acid, the precursor of prostanoids, is metabolized to prostaglandin H2 (PGH2) by cyclooxygenase enzyme (COX) in a two-step process. The synthesized PGH2 is converted to prostaglandins and other prostanoids by variable synthase enzymes. Prostanoids play a role in many inflammatory processes. Therefore, COX is the key enzyme of the arachidonic acid cascade, and COX inhibition is a helpful way to reduce inflammation, pain, and fever caused by prostaglandins. Cyclooxygenase exists in three isoforms, COX-1, COX-2, and COX-3. COX-1 participates in physiological functions, whereas COX-2 mediates pathological processes [1]. Much less is known about COX-3, which is expressed in the cerebral cortex and cardiac tissue and regulates fever and pain [2]. Non-steroidal anti-inflammatory drugs (NSAIDs) demonstrate their anti-inflammatory effects by inhibiting both COX-1 and COX-2 isoforms, which causes them to miss out on advantages of COX-1 functions such as stomach protection and renal hemodynamics. Hence, non-selective inhibition of the COX enzyme leads to side effects such as gastrointestinal ulcers, kidney injuries, etc.
Moreover, these two isoforms have some structural differences as well. The replacement of Val523, Val434, and Arg513 in COX-2 instead of Ile523, Ile434, and His513 in COX-1, respectively, results in structural modification. These three amino acid alterations lead to a larger space in the COX-2 isozyme, called the secondary pocket. Thus, compounds containing crucial pharmacophores, which could occupy the secondary pocket and form interactions with essential amino acids in COX-2, may selectively inhibit COX-2 rather than COX-1 [3].
In contrast to NSAIDs, selective COX-2 inhibitors diminish undesirable COX-2 inflammatory mediators without interrupting COX-1 housekeeping functions. Besides, COX-2 overexpression was reported in different diseases such as many cancers (breast, colorectal, and prostate cancer) and neurodegenerative diseases like amyotrophic lateral sclerosis (ALS), Alzheimer’s, and Parkinson’s disease [4,5,6,7,8]. In addition, it has been proved that COX-2 is one of the enzymes that contribute to the increment of pro-inflammatory metabolites, causing intensifying tissue morbidity in COVID-19 [9]. These findings suggest that COX-2 could be one of the therapeutic targets in these pathophysiological disorders [10,11,12]. Accordingly, plenty of studies proved that COX-2 inhibition plays a beneficial role in the treatment of such diseases. Owing to a wide variety of applications, the discovery of potent COX-2 inhibitors with a safe profile of adverse effects is noticeable.
There is a wide variety of COX-2 inhibitors. Generally, these compounds contain two vicinal phenyl rings on a central system which can be carbo/heterocyclic (tricyclics) or acyclic [13, 14]. A pharmacophore group such as methanesulfonyl, sulfonamide, or azido at the para-position of one of the phenyl rings plays an important role in COX-2 selectivity [15]. These substituents could insert into the secondary pocket that exists in the COX-2 isozyme. This pocket includes three crucial amino acids: Arg513, His90, and Val523. The pharmacophore group of COX-2 inhibitors forms hydrogen bonding with essential amino acids after inserting into the secondary pocket, which leads to selective inhibition of COX-2.
Different types of central heterocyclic or carbocyclic ring systems, such as 4-, 5- and 6-membered rings and fused bicyclic, tricyclic, and spiro ring systems, are seen as a central core of COX-2 inhibitors [16,17,18,19,20,21,22,23]. Acyclic COX-2 inhibitors (non-tricyclics) contain a two-membered (olefins) or three-membered (chalcones) chain structure, which is the essential point for sub-classification of these compounds [23,24,25,26,27,28,29]. In addition, in order to discover novel templates of COX-2 inhibitors, many researchers introduced small peptide analogs of COX-2 inhibitors [30, 31]. Furthermore, conjugating COX-2 inhibitors with some moieties, such as nitric oxide-releasing and ferrocene, is one strategy to afford molecules with reduced side effects and more parallel biological effects [32, 33]. Some hybrid molecules named COX/LOX inhibitors provide improved anti-inflammatory, cardiovascular, and gastrointestinal safety profiles [34, 35].
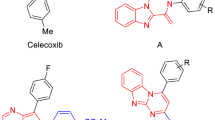
According to the literature, a class of 1,1-diphenyl-2-(4-methylsulfonylphenyl)-2-alkyl-1-ethenes was prepared and evaluated among acyclic compounds. In this group, selectivity and COX-2 inhibitory potency depend on 2-alkyl chain length; the namely n-butyl substituent (Fig. 1A) exhibited high potency and selectivity even better than celecoxib (IC50 = 0.014 µM, selectivity index (S.I.) > 7142) [36].
In the previous study, an imidazo[1,2-a]pyridine scaffold was chosen for COX-2 inhibitory activity; a new series of 2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridine with different substituents on C-3 of the central ring was reported [37]. The results of in vitro studies indicated that COX-2 inhibitory activity was impressed by the nature and size of mannich base on C-3 of imidazo[1,2-a]pyridine ring. The compound with morpholine ring at C-3 showed the highest potency and selectivity (Fig. 1B) (IC50 = 0.07 μM, selectivity index = 217.1). Imidazo[1,2-a]pyridine is one of the most popular bicyclic heterocyclic pharmacophores due to its broad spectrum of biological activities such as anticancer, anticonvulsant, hypnotic, antimycobacterial, antimicrobial, antiviral, analgesic, and antidiabetic are known as a privileged scaffold in medicinal chemistry [38,39,40]. Hence, to design more efficient and selective COX-2 inhibitors rationally, we decided to modify the previous 2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridine derivatives. Accordingly, the mannich base group at C-3 of the central ring was substituted with the phenylamino group to investigate the effects of an phenylamino group and different substituents on this phenyl ring at this position. Consequently, the present study described some novel 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine derivatives in order to evaluate in vitro COX-1/COX-2 inhibition and in vivo analgesic activities.
Results and discussion
Chemistry
The target 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine derivatives were synthesized via the route outlined in Scheme 1.
Initially, α-bromo-4-(methylsulfonyl)acetophenone 1 was prepared according to the literature procedure [34]. The α-bromo-4-(methylsulfonyl)acetophenone 1 and appropriate 4-substituted aniline 2 in the presence of NaHCO3 in anhydrous MeOH were reacted to afford 1-(4-(methylsulfonyl)phenyl)-2-(phenylamino)ethan-1-ones (3a–e) [41]. Condensation of 3 with different 2-aminopyridines in i-PrOH at 80 °C gave final 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine derivatives (5a–t) in good yields [42].
The structures of all derivatives were characterized by IR, LC-Ms and NMR spectroscopy. The purity of compounds was confirmed by TLC and HPLC. The IR spectra indicated the SO2Me peak at wavenumbers about 1150 and 1300 cm−1. Also, the NH group appeared in about 3200–3450 cm−1 wavenumber. Besides the elimination of the carbonyl peak of the intermediate, these two results confirmed the synthesis of desired compounds. The (M + 1)+ peaks in LC-Ms revealed the formation of hydrogen adduct during the ionization in the mass spectroscopy and comprised the synthesis of compounds. The NMR data, with proper chemical shifts and integrals, could characterize the structures. For example, the deshield doublet of doublets in the chemical shifts 7.9–8.3 ppm exhibited the hydrogens of methylsulfonylphenyl ring. The singlet peak of the NH group is mostly presented above 8.0 ppm. In the aliphatic part of the spectra, the singlet peak with the chemical shift around 3.2 ppm indicated the presence of SO2Me in the structures. TLC and HPLC results showed that all compounds were pure and stable.
Biological evaluation
In vitro cyclooxygenase (COX) inhibition assays
The inhibitory activities of novel 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amines against COX-1 and COX-2 were evaluated by in vitro assay. As shown in Table 1, all compounds were selective COX-2 inhibitors with selectivity indices of 42.3–508.6 and COX-2 IC50 values of 0.07–0.39 µM. Compound 8-methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5n) with COX-2 IC50 value of 0.07 µM and selectivity index of 508.6 exhibited highest inhibitory potency and selectivity.
Structurally the synthesized compounds 5a–t could be categorized into five groups based on the type of substituent at the para position of the phenylamino ring: hydrogen, 4-fluoro, 4-chloro, 4-methyl, and 4-methoxy derivatives (5a–d, 5e–h, 5i–l, 5m–p, and 5q–t) to evaluate steric, electronic and hydrophobic effects on activities. In each group, hydrogen was replaced with a methyl substituent at different positions of the imidazopyridine ring to examine hydrophobic and steric parameters around this ring.
As shown in Table 1, replacing fluorine at the para position of phenylamino improved both potency and selectivity compared to other groups. It may be explained by the ability of fluorine to form hydrogen bonding with amino acids of the active site. Among compounds having 4-F and 4-OMe (in F and OMe groups), derivatives having methyl on imidazopyridine ring (5f, 5g, 5h, 5r, 5s, and 5t) and derivatives without methyl on imidazopyridine ring (5e and 5q) exhibited the highest and the lowest COX-2 IC50 and selectivity index respectively in each group. It seems that methyl substituent on the imidazopyridine ring leads to better interaction of F and OMe on the phenylamino ring due to the more appropriate orientation of these molecules into the active site of COX-2.
In this series, introducing a suitable substituent, especially on C-8 of imidazo[1,2-a]pyridine ring, enhances selectivity on COX-2 isozyme (except in fluoro and chloro group). This may be explained by steric hindrance during the interaction of the molecule with COX-1.
These results indicated that the 2-phenyl-N-phenylimidazo[1,2-a]pyridin-3-amine structure was a suitable scaffold for COX-1/2 inhibition, and adding SO2Me pharmacophore at the para position of C-2 phenyl ring enhanced COX-2 potency and selectivity.
In vivo evaluation of analgesic effects
The in vivo formalin test was performed to assess the analgesic activity of synthesized compounds. The result was compared with celecoxib as the reference drug. The results have been summarized in Table 1. A significant reduction in the AUC of pain score was shown in groups treated with 5a, 5r, 5s, and 5t (p < 0.001), 5k (p < 0.01), and 5d, 5h, 5i, and 5q (p < 0.05), compared with the control group. There was at least one efficient compound in each category. In OMe substituted compounds, all the compounds (5q, 5r, 5s, and 5t) were active enough to reduce the AUC of pain score (p < 0.05). Surprisingly, even compound 5q, which presented poor COX-2 inhibition compared to other potent compounds, showed significant antinociceptive activity. It seems some pharmacokinetic factors are affected differently in vitro and in vivo activities of this molecule.
Molecular modeling studies
The binding affinity of each compound to the COX-2 active site showed in Table 2. The results indicated that most of the compounds have a high affinity to the active site, even more than the standard compounds, celecoxib and SC-558.
The docking poses of two potent and selective compounds depicted the SO2Me group interacting with essential amino acids of the COX-2 secondary pocket. As shown in Fig. 2, the oxygen atoms of SO2Me of each molecule can form hydrogen bonds with NH of Arg513, His90, and Phe518 (distances = 2.3, 3.5, and 2.3 Å for the compound 5n; 2.1, 3.1, and 2.2 Å for compound 5h). The imidazopyridine moiety of molecules form hydrogen bonds with NH of Arg120 within nitrogen atoms of the ring (distances = 4.2 Å for both compounds 5h and 5n). Further, fluoro substituent of 5h interacts with NH of Gly526 (distance = 2.2 Å). These docking studies show that hydrophobic side chains of Trp387, Leu531, Val 349, Leu384, Tyr385, and Met522 residues surround hydrophobic moieties of molecules such as phenyl or pyridine rings which may undergo hydrophobic interactions. The docking results also revealed that 5h and 5n positions in the COX-2 active site provided a suitable orientation. These molecules were perfectly superimposed on SC-558, a selective inhibitor in a complex with COX-2 (Fig. 3).
As mentioned in the introduction, due to the presence of a secondary pocket in COX-2, the compounds containing the pharmacophore moiety, such as SO2Me, could insert the secondary pocket and inhibit COX-2 selectively. Therefore, based on the docking study results, it is expected that the designed compounds would be able to inhibit the COX-2 enzyme selectively.
The re-docking of the co-crystalized ligand SC-558 confirmed the validation of the molecular modeling study. The RMS over 21 pairs was 0.33. The SC-558 revealed high-affinity binding (−10.5 Kcal/mol) to the COX-2 active site.
Conclusion
In conclusion, a series of 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine having different substituents at the para position of the N-phenyl ring were introduced as COX-2 inhibitors. These compounds were synthesized through two-step reactions with high purity and yields. The docking studies demonstrated that all designed compounds possess well docking scores; even 5a, 5b, 5c, 5e, 5f and 5h showed higher affinity than celecoxib and SC-558. It should be noted that the biological assays were in accordance with the docking score; most compounds showed high potencies and selectivity indices against the COX-2 isozyme. The 8-methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5n) was the most active compound in enzyme inhibition assay, even more, selective than reference drug celecoxib (S.I. = 508). The formalin test indicated that N-(4-methoxyphenyl)-8-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5r) displayed the highest analgesic activity. In addition, compounds 5a, 5s, and 5t, with significant reductions in AUC of pain scores, were also promising compounds in the formalin test.
Materials and methods
General methods
All chemicals handled in the preparations were purchased from Merck and/or Sigma-Aldrich. 1H and 13C NMR spectra were recorded on a Brucker FT-400 MHz instrument (Brucker Biosciences, USA) using Chloroform-D and DMSO-d6 as solvents and tetramethylsilane (TMS) as an internal standard. Melting points were determined with a Thomas–Hoover capillary apparatus. Infrared spectra were obtained using a Perkin Elmer Model 1420 spectrometer. The mass spectral measurements were observed on a 6410 Agilent LC-MS triple quadrupole mass spectrometer (LC-MS) with an electrospray ionization (ESI) interface. Microanalyses determined for C, H and N were within ±0.4% of the theoretical values, using the elemental combustion system, Costech 4010. A Waters 2695 HPLC system (Milford, USA) employed consisted of a Waters 2695 pump, Rheodyne 7125 injector and a 2996 PDA detector.
Chemical synthesis
General procedure for the synthesis of 1-(4-(methylsulfonyl)phenyl)-2-(phenylamino)ethan-1-ones (3)
A mixture of α-bromo-4-(methylsulfonyl)acetophenone 1 (7 mmol) and 4-substituted-aniline 2 (7 mmol) in anhydrous methanol (10 ml) and in the presence of two equivalents of sodium hydrogen carbonate was stirred at room temperature for 16 h. The reaction mixture was filtered out and washed with cold MeOH and water. The crude was used in the next step without any purification.
1-(4-(methylsulfonyl)phenyl)-2-(phenylamino)ethan-1-one (3a)
Yield, 91%; Yellow powder; mp: 156–158 °C; IR (KBr disk): νcm−1 1155, 1296 (SO2), 1700 (C=O), 3396 (NH); LC-MS (ESI) m/z: 288 ([M-H]−, 100).
2-((4-fluorophenyl)amino)-1-(4-(methylsulfonyl)phenyl)ethan-1-one (3b)
Yield, 89%; Yellow powder; mp: 166–168 °C; IR (KBr disk): νcm−1 1142, 1309 (SO2), 1692 (C=O), 3364 (NH); LC-MS (ESI) m/z: 306 ([M-H]−, 100).
2-((4-chlorophenyl)amino)-1-(4-(methylsulfonyl)phenyl)ethan-1-one (3c)
Yield, 87%; Yellow powder; mp: 171–173 °C; IR (KBr disk): νcm−1 1142, 1308 (SO2), 1692 (C=O), 3355 (NH); LC-MS (ESI) m/z: 322 ([M-H]−, 100).
1-(4-(methylsulfonyl)phenyl)-2-(p-tolylamino)ethan-1-one (3d)
Yield, 86%; Yellow powder; mp: 153–154 °C; IR (KBr disk): νcm−1 1152, 1297 (SO2), 1683 (C=O), 3390 (NH); LC-MS (ESI) m/z: 302 ([M-H]−, 100).
2-((4-methoxyphenyl)amino)-1-(4-(methylsulfonyl)phenyl)ethan-1-one (3e)
Yield, 81%; dark yellow powder; mp: 149–151 °C; IR (KBr disk): νcm−1 1154, 1297 (SO2), 1675 (C=O), 3361 (NH); LC-MS (ESI) m/z: 318 ([M-H]−, 100).
General procedure for the synthesis of 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine derivatives (5)
An appropriate derivative of 3 (1.73 mmol), 2-aminopyridine derivatives (1.73 mmol), ZnI2 (0.52 mmol), 4 Å MS (850 mg), and i-PrOH (8.5 ml) were added, and the mixture was stirred at 80 °C. After completing the reaction, the mixture was cooled to room temperature and then filtered and washed with water and cool i-PrOH to obtain 20 different derivatives. For further purification, recrystallization with ethanol 96% was carried out.
2-(4-(Methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine (5a)
Yield, 79%; yellow powder; mp: 189 °C (decomposed); IR (KBr disk): νcm−1 1158, 1299 (SO2), 1682 (C=N), 3404 (NH); 1H NMR (DMSO-d6): δ ppm 3.25 (s, 3H, SO2Me), 6.56 (d, 2H, J = 7.6 Hz, phenyl H2 and H6), 6.74 (t, 1H, J = 7.6 Hz, phenyl H4), 6.90 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 6.99 (t, 2H, J = 7.6 Hz, phenyl H3 and H5), 7.25 (t, 1H, J = 6.8 Hz, imidazopyridine H7), 7.42 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.65 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.99 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.12 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.16 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 43.57, 113.26, 113.49, 117.67, 117.84, 119.32, 123.80, 127.25, 127.67, 127.97, 129.31, 129.44. 130.09, 140.50, 144.72, 146.77; LC-MS (ESI) m/z: 364 ([M + H]+, 100); Anal. Calcd. For C20H17N3O2S: C, 66.10; H, 4.72; N, 11.56. Found: C, 65.88; H, 4.74; N, 11.68.
8-Methyl-2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine (5b)
Yield, 71%; yellow powder; mp: 261–263 °C; IR (KBr disk): νcm−1 1158, 1269 (SO2), 1631 (C=N), 3373 (NH); 1H NMR (CDCl3): δ ppm 2.62 (s, 3H, CH3), 2.96 (s, 3H, SO2Me), 5.61 (s, 1H, NH), 6.53 (d, 2H, J = 7.6 Hz, phenyl H2 and H6), 6.67 (t, 1H, J = 6.8 Hz phenyl H4), 6.81 (t, 1H, J = 7.2 Hz, imidazopyridine H6), 7.00 (d, 1H, J = 6.8 Hz, imidazopyridine H7), 7.15 (t, 2H, J = 7.6 Hz, phenyl H3 and H5), 7.67 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.83 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.18 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 16.65, 43.99, 113.20, 113.48, 119.25, 121.29, 121.56, 124.76, 127.23, 127.40, 127.72, 130.07, 135.61, 139.18, 139.57, 142.85, 145.71; LC-MS (ESI) m/z: 378 ([M + H]+, 100); Anal. Calcd. For C21H19N3O2S: C, 66.82; H, 5.07; N, 11.13. Found: C, 66.76; H, 4.96; N, 11.24.
7-Methyl-2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine (5c)
Yield, 78%; yellow powder; mp: 239–241 °C; IR (KBr disk): νcm−1 1162, 1317 (SO2), 1661 (C=N), 3235 (NH); 1H NMR (DMSO-d6): δ ppm 2.38 (s, 3H, CH3), 3.20 (s, 3H, SO2Me), 6.51 (d, 2H, J = 7.6 Hz, phenyl H2 and H6), 6.73 (t, 1H, J = 7.2 Hz, phenyl H4), 6.8 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 7.14 (t, 2H, J = 7.6 Hz, phenyl H3 and H5), 7.43 (s, 1H, imidazopyridine H8), 7.86 (d, 1H, J = 6.8 Hz imidazopyridine H5), 7.93 (d, 2H, J = 8.0 Hz, methylsulfonylphenyl H2 and H6), 8.28 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.32 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 21.32, 43.99, 113.45, 115.70, 116.10, 119.23, 120.59, 123.05, 127.14, 127.69, 130.06, 135.72, 136.89, 139.23, 139.50, 142.96, 145.75; LC-MS (ESI) m/z: 378 ([M + H]+, 100); Anal. Calcd. For C21H19N3O2S: C, 66.82; H, 5.07; N, 11.13. Found: C, 66. 69; H, 5.11; N, 11.19.
5-Methyl-2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine (5d)
Yield, 72%; yellow powder; mp: 210 °C (decomposed); IR (KBr disk): νcm−1 1162, 1326 (SO2), 1668 (C=N), 3361 (NH); 1H NMR (DMSO-d6): δ ppm 2.66 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.66 (d, 2H, J = 6.8 Hz, phenyl H2 and H6), 6.70 (t, 1H, J = 7.6 Hz, phenyl H4), 6.81 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 7.15 (t, 2H, J = 7.2 Hz, phenyl H3 and H5), 7.23 (t, 1H, J = 7.2 Hz, imidazopyridine H7), 7.50 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.91 (d, 2H, J = 8.8 Hz, methylsulfonylphenyl H2 and H6), 8.22 (s, 1H, NH), 8.31 (d, 2H, J = 8.8 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 18.25, 43.91, 113.24, 114.26, 116.09, 118.78, 121.66, 126.57, 127.38, 127.62, 130.23, 136.66, 137.77, 139.10, 139.71, 144.29, 148.15; LC-MS (ESI) m/z: 378 ([M + H]+, 100); Anal. Calcd. For C21H19N3O2S: C, 66.82; H, 5.07; N, 11.13. Found: C, 66.91; H, 5.09; N, 11.02.
N-(4-Fluorophenyl)-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5e)
Yield, 56%; cream powder; mp: 238 °C (decomposed); IR (KBr disk): νcm−1 1170, 1330 (SO2), 1648 (C=N), 3263 (NH); 1H NMR (DMSO-d6): δ ppm 3.22 (s, 3H, SO2Me), 6.50–6.54 (m, 2H, phenyl H2 and H6), 6.95–7.02 (m, 3H, phenyl H3 and H5, imidazopyridine H6), 7.36 (t, 1H, J = 7.2 Hz, imidazopyridine H7), 7.67 (d, 1H, J = 9.2 Hz, imidazopyridine H8), 7.95 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.00 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 8.30 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.36 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 43.97, 113.26, 114.48, 114.56, 116.47, 116.70, 117.94, 121.17, 123.78, 126.36, 127.24, 127.76, 136.05, 138.99, 139.73, 142.08, 142.57, 155.17, 157.49; LC-MS (ESI) m/z: 382 ([M + H]+, 100); Anal. Calcd. For C20H16FN3O2S: C, 62.98; H, 4.23; N, 11.02. Found: C, 63.15; H, 4.21; N, 11.08.
N-(4-Fluorophenyl)-8-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5f)
Yield, 47%; white powder; mp: 224–226 °C; IR (KBr disk): νcm−1 1144, 1308 (SO2), 1621 (C=N), 3212 (NH); 1H NMR (DMSO-d6): δ ppm 2.59 (s, 3H, CH3), 3.22 (s, 3H, SO2Me), 6.50–6.53 (m, 2H, phenyl H2 and H6), 6.87 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 6.99 (t, 2H, J = 8.8 Hz, phenyl H3 and H5), 7.17 (d, 1H, J = 6.8 Hz, imidazopyridine H7), 7.85 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.96 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.31 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.35 (s, 1H, NH); 13C NMR (DMSO): δ ppm 16.63, 44.00, 113.25, 114.47, 114.54, 116.44, 116.67, 121.54, 124.79, 127.22, 127.42, 127.75, 135.59, 139.13, 139.60, 142.17, 142.87, 155.13, 157.46; LC-MS (ESI) m/z: 396 ([M + H]+, 100); Anal. Calcd. For C21H18FN3O2S: C, 63.78; H, 4.59; N, 10.63. Found: C, 63.61; H, 4.62; N, 10.57.
N-(4-Fluorophenyl)-7-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5g)
Yield, 52%; yellow powder; mp: 238–240 °C; IR (KBr disk): νcm−1 1152, 1310 (SO2), 1645 (C=N), 3215 (NH); 1H NMR (DMSO-d6): δ ppm 2.38 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.48–6.52 (m, 2 H, phenyl H2 and H6), 6.80 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 6.99 (t, 2H, J = 8.8 Hz, phenyl H3 and H5), 7.43 (s, 1H, imidazopyridine H8), 7.88 (d, 1H, J = 7.2 Hz, imidazopyridine H5), 7.93 (d, 2H, J = 8.8 Hz, methylsulfonylphenyl H2 and H6), 8.27 (d, 2H, J = 8.8 Hz, methylsulfonylphenyl H3 and H5), 8.30 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 21.31, 43.99, 114.41, 114.49, 115.76, 116.11, 116.44, 116.66, 120.74, 123.02, 127.11, 127.71, 135.69, 136.93, 139.16, 139.54, 142.21, 142.96, 155.12, 157.44; LC-MS (ESI) m/z: 396 ([M + H]+, 100); Anal. Calcd. For C21H18FN3O2S: C, 63.78; H, 4.59; N, 10.63. Found: C, 63.88; H, 4.55; N, 10.66.
N-(4-Fluorophenyl)-5-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5h)
Yield, 60%; white powder; mp: 230 °C (decomposed); IR (KBr disk): νcm−1 1153, 1315 (SO2), 1648 (C=N), 3343 (NH); 1H NMR (DMSO-d6): δ ppm 2.68 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.48 (m, 2H, phenyl H2 and H6), 6.86 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 6.99 (t, 2H, J = 8.8 Hz, phenyl H3 and H5), 7.24 (t, 1H, J = 8.4 Hz, imidazopyridine H7), 7.51 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.93 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.11 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.33 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 18.83, 43.92, 114.25, 116.08, 116.56, 116.78, 121.96, 126.53, 127.37, 127.63, 130.11, 136.69, 137.72, 139.09, 139.70, 141.21, 144.28, 154.71, 157.03; LC-MS (ESI) m/z: 396 ([M + H]+, 100); Anal. Calcd. For C21H18FN3O2S: C, 63.78; H, 4.59; N, 10.63. Found: C, 63.59; H, 4.63; N, 10.71.
N-(4-Chlorophenyl)-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5i)
Yield, 55%; yellow powder; mp: 226–228 °C; IR (KBr disk): νcm−1 1154, 1307 (SO2), 1669 (C=N), 3333 (NH); 1H NMR (DMSO-d6): δ ppm 3.22 (s, 3H, SO2Me), 6.53 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 6.97 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 7.19 (d, 2H, J = 8.8 Hz phenyl H3 and H5), 7.37 (t, 1H, J = 8.4 Hz, imidazopyridine H7), 7.68 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.96 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.01 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 8.29 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.54 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 43.97, 113.36, 115.08, 117.96, 120.47, 122.81, 123.77, 126.45, 127.25, 127.80, 129.86, 136.15, 138.90, 139.80, 142.66, 144.64; LC-MS (ESI) m/z: 398 ([M + H]+, 100), 400 (M + 3, 32%); Anal. Calcd. For C20H16ClN3O2S: C, 60.38; H, 4.05; N, 10.56. Found: C, 60.45; H, 4.01; N, 10.58.
N-(4-Chlorophenyl)-8-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5j)
Yield, 59%; creamy-yellowish powder; mp: 199–201 °C; IR (KBr disk): νcm−1 1141, 1304 (SO2), 1670 (C=N), 3354 (NH); 1H NMR (DMSO-d6): δ ppm 2.58 (s, 3H, CH3), 3.22 (s, 3H, SO2Me), 6.52 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 6.88 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 7.18 (m, 3H, imidazopyridine H7, phenyl H3 and H5), 7.85 (d, 1H, J = 6.4 Hz, imidazopyridine H5), 7.96 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.30 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.54 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 16.64, 44.00, 113.34, 115.06, 120.85, 121.51, 122.74, 124.88, 127.23, 127.46, 127.78, 129.84, 135.68, 139.03, 139.67, 142.96, 144.73; LC-MS (ESI) m/z: 412 ([M + H]+, 100), 414 ([M + H + 2]+, 32%); Anal. Calcd. For C21H18ClN3O2S: C, 61.24; H, 4.40; N, 10.20. Found: C, 61.01; H, 4.44; N, 10.30.
N-(4-Chlorophenyl)-7-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5k)
Yield, 71%; yellow powder; mp: 238 °C (decomposed); IR (KBr disk): νcm−1 1150, 1299 (SO2), 1670 (C=N), 3201 (NH); 1H NMR (DMSO-d6): δ ppm 2.38 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.51 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 6.81 (d, 1 H, J = 6.8 Hz, imidazopyridine H6), 7.18 (d, 2H, J = 8.8 Hz, phenyl H3 and H5), 7.44 (s, 1H, imidazopyridine H8), 7.88 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.94 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.25 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.50 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 21.31, 43.99, 115.02, 115.85, 116.13, 120.04, 122.71, 123.01, 127.12, 127.75, 129.84, 135.78, 137.04, 139.06, 139.60, 143.05, 144.77; LC-MS (ESI) m/z: 412 ([M + H]+, 414 ([M + H + 2]+, 32%); Anal. Calcd. For C21H18ClN3O2S: C, 61.24; H, 4.40; N, 10.20. Found: C, 61.20; H, 4.37; N, 10.26.
N-(4-Chlorophenyl)-5-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5l)
Yield, 73%; yellow powder; mp: 249 °C (decomposed); IR (KBr disk): νcm−1 1148, 1316 (SO2), 1655 (C=N), 3372 (NH); 1H NMR (DMSO-d6): δ ppm 2.65 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.50 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 6.68 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 7.18–7.26 (m, 3H, phenyl H3 and H5, imidazopyridine H7), 7.50 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.92 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.27 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5), 8.40 (s, 1H, NH); 13C NMR (DMSO-d6): δ ppm 18.81, 43.90, 114.41, 116.14, 121.14, 122.28, 126.69, 127.34, 127.69, 129.35, 130.03, 136.56, 137.76, 138.92, 139.82, 144.37, 147.07; LC-MS (ESI) m/z: 412 ([M + H]+, 100), ([M + H + 2]+, 32%); Anal. Calcd. For C21H18ClN3O2S: C, 61.24; H, 4.40; N, 10.20. Found: C, 61.16; H, 4.43; N, 10.16.
2-(4-(Methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5m)
Yield, 63%; yellow powder; mp: 198–200 °C; IR (KBr disk): νcm−1 1153, 1313 (SO2), 1635 (C=N), 3335 (NH); 1H NMR (DMSO-d6): δ ppm 2.15 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 6.42–6.44 (d, 2H, J = 8.0 Hz, phenyl H3 and H5), 6.93–6.97 (m, 3H, imidazopyridine H6, phenyl H2 and H6), 7.33–7.37 (t, 1H, J = 7.6 Hz, imidazopyridine H7), 7.65–7.67 (d, 1H, J = 9.2 Hz, imidazopyridine H8), 7.93–7.97 (m, 3H, methylsulfonylphenyl H2 and H6, imidazopyridine H5), 8.21 (s, 1H, NH), 8.29–8.31 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 20.55, 43.97, 113.13, 113.51, 117.89, 121.42, 123.79, 126.25, 127.23, 127.72, 127.90, 130.51, 135.99, 139.11, 139.63, 142.48, 143.18; LC-MS (ESI) m/z: 378 ([M + H]+, 100); Anal. Calcd. For C21H19N3O2S: C, 66.82; H, 5.07; N, 11.13. Found: C, 66.98; H, 5.11; N, 11.19.
8-Methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5n)
Yield, 59%; dark yellow powder; mp: 200–202 °C; IR (KBr disk): νcm−1 1156, 1315 (SO2), 1632 (C=N), 3370 (NH); 1H NMR (DMSO-d6): δ ppm 2.15 (s, 3H, 4-CH3), 2.58 (s, 3H, 8-CH3), 3.21 (s, 3H, SO2Me), 6.42 (d, 2H, J = 8.0 Hz, phenyl H3 and H5), 6.85 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 6.95 (d, 2H, J = 8.0 Hz, phenyl H2 and H6), 7.15 (d, 1H, J = 6.8 Hz, imidazopyridine H7), 7.81 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.94 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.20 (s, 1H, NH), 8.31 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 16.64, 20.55, 44.01, 113.10, 113.50, 121.55, 121.79, 124.68, 127.22, 127.36, 127.69, 127.83, 130.48, 135.52, 139.25, 139.49, 142.78, 143.27; LC-MS (ESI) m/z: 392 ([M + H]+, 100); Anal. Calcd. For C22H21N3O2S: C, 67.50; H, 5.41; N, 10.73. Found: C, 67.41; H, 4.39; N, 10.78.
7-Methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5o)
Yield, 61%; creamy powder; mp: 274 °C (decomposed); IR (KBr disk): νcm−1 1158, 1320 (SO2), 1648 (C=N), 3224 (NH); 1H NMR (DMSO-d6): δ ppm 2.16 (s, 3H, 4-CH3), 2.38 (s, 3H, 7-CH3), 3.20 (s, 3H, SO2Me), 6.41 (d, 2H, J = 8.0 Hz, phenyl H3 and H5), 6.79 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 6.94 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 7.42 (s, 1H, imidazopyridine H8), 7.83 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.92 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.15 (s, 1H, NH), 8.27 (d, 2H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 19.47, 20.23, 42.91, 112.38, 114.53, 114.99, 119.91, 121.95, 126.02, 126.58, 126.73, 129.39, 134.53, 135.71, 138.21, 138.35, 141.80, 142.23; LC-MS (ESI) m/z: 392 ([M + H]+, 100); Anal. Calcd. For C22H21N3O2S: C, 67.50; H, 5.41; N, 10.73. Found: C, 67.64; H, 5.42; N, 10.66.
5-Methyl-2-(4-(methylsulfonyl)phenyl)-N-(p-tolyl)imidazo[1,2-a]pyridin-3-amine (5p)
Yield, 63%; yellow powder; mp: 330 °C (decomposed); IR (KBr disk): νcm−1 1156, 1321 (SO2), 1648 (C=N), 3390 (NH); 1H NMR (DMSO-d6): δ ppm 2.15 (s, 3H, CH3), 2.66 (s, 3H, CH3), 3.20 (s, 3H, SO2Me), 6.43 (d, 2H, J = 8.0 Hz, phenyl H3 and H5), 6.65 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 6.95 (d, 2H, J = 8.0 Hz, phenyl H2 and H6), 7.22 (t, 1H, J = 7.2 Hz, imidazopyridine H7), 7.49 (d, 1H, J = 9.6 Hz, imidazopyridine H8), 7.90 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.04 (s, 1H, NH), 8.30 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 18.82, 20.53, 43.81, 114.19, 116.06, 117.43, 122.06, 126.50, 127.23, 127.35, 127.59, 128.10, 130.67, 136.69, 137.68, 139.66, 144.26, 145.81; LC-MS (ESI) m/z: 392 ([M + H]+, 100); Anal. Calcd. For C22H21N3O2S: C, 67.50; H, 5.41; N, 10.73. Found: 67.59; H, 5.38; N, 10.75.
N-(4-Methoxyphenyl)-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5q)
Yield, 38%; dark yellow powder; mp: 103–105 °C; IR (KBr disk): νcm−1 1160, 1320 (SO2), 1639 (C=N), 33230 (NH); 1H NMR (CDCl3): δ ppm 3.22 (s, 3H, SO2Me), 3.63 (s, 3H, OCH3), 6.47 (d, 2H, J = 8.8 Hz, phenyl H3 and H5), 6.76 (d, 2H, J = 9.2 Hz, phenyl H2 and H6), 6.95 (t, 1H, J = 6.8 Hz, imidazopyridine H6), 7.34 (t, 1H, J = 8.0 Hz, imidazopyridine H7), 7.65 (d, 1H, J = 9.2 Hz, imidazopyridine H8), 7.94 (d, 1H, J = 8.8 Hz, methylsulfonylphenyl H2 and H6), 7.98 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 8.07 (s, 1H, NH), 8.31 (d, 1H, J = 8.8 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (CDCl3): δ ppm 43.98, 55.67, 113.09, 114.48, 117.89, 121.89, 123.81, 126.21, 127.22, 127.71, 135.90, 139.15, 139.60, 142.42, 153.04; LC-MS (ESI) m/z: 394 ([M + H]+, 100); Anal. Calcd. For C21H19N3O3S: C, 64.11; H, 4.87; N, 10.68. Found: C, 63.93; H, 4.89; N, 10.76.
N-(4-Methoxyphenyl)-8-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5r)
Yield, 41%; white-creamy powder; mp: 229–231 °C; IR (KBr disk): νcm−1 1151, 1314 (SO2), 1627 (C=N), 3252 (NH); 1H NMR (DMSO-d6): δ ppm 2.57 (s, 3H, CH3), 3.21 (s, 3H, SO2Me), 3.62 (s, 3H, OCH3), 6.46 (d, 2H, J = 8.8 Hz, phenyl H3 and H5), 6.76 (d, 2H, J = 8.8 Hz, phenyl H2 and H6), 6.85 (t, 1H, J = 7.2 Hz, imidazopyridine H6), 7.15 (d, 1H, J = 6.8 Hz, imidazopyridine H7), 7.83 (d, 1H, J = 6.8 Hz, imidazopyridine H5), 7.95 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.07 (s, 1H, NH), 8.33 (d, 1H, J = 8.8 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 16.64, 44.01, 55.67, 113.07, 114.47, 115.54, 121.57, 122.27, 124.64, 127.21, 127.70, 135.43, 139.28, 139.46, 142,73, 153.00; LC-MS (ESI) m/z: 408 ([M + H]+, 100); Anal. Calcd. For C22H21N3O3S: C, 64.85; H, 5.19; N, 10.31. Found: C, 65.01; H, 5.15; N, 10.25.
N-(4-Methoxyphenyl)-7-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5s)
Yield, 44%; white powder; mp: 138–140 °C; IR (KBr disk): νcm−1 1155, 1307 (SO2), 1648 (C=N), 3227 (NH); 1H NMR (DMSO-d6): δ ppm 2.37 (s, 1H, CH3), 3.21 (s, 3H, SO2Me), 3.63 (s, 3H, OCH3), 6.46 (d, 2H, J = 8.8 Hz, phenyl H3 and H5), 6.75–6.77 (m, 3H, phenyl H2 and H6, imidazopyridine H6), 7.41 (s, 1H, imidazopyridine H8), 7.85 (d, 1H, J = 7.2 Hz, imidazopyridine H5), 7.93 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H2 and H6), 8.03 (s, 1H, NH), 8.29 (d, 1H, J = 8.4 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 21.31, 44.00, 55.67, 114.42, 115.55, 116.06, 121.47, 123.05, 127.10, 127.67, 135.54, 136.75, 139.33, 139.40, 142.83, 152.99; LC-MS (ESI) m/z: 408 ([M + H]+, 100); Anal. Calcd. For C22H21N3O3S: C, 64.85; H, 5.19; N, 10.31. Found: C, 64.98; H, 5.14; N, 10.24.
N-(4-Methoxyphenyl)-5-methyl-2-(4-(methylsulfonyl)phenyl)imidazo[1,2-a]pyridin-3-amine (5t)
Yield, 50%; yellow-orange powder; mp: 156 °C (decomposed); IR (KBr disk): νcm−1 1152, 1313 (SO2), 1641 (C=N), 3365 (NH); 1H NMR (DMSO-d6): δ ppm 2.68 (s, 1H, CH3), 3.21 (s, 3H, SO2Me), 3.62 (s, 3H, OCH3), 6.38 (d, 2H, J = 8.4 Hz, phenyl H3 and H5), 6.64 (d, 1H, J = 6.8 Hz, imidazopyridine H6), 6.77 (d, 2H, J = 8.4 Hz, phenyl H2 and H6), 7.21 (t, 1H, J = 7.2 Hz, imidazopyridine H7), 7.48 (d, 1H, J = 8.8 Hz, imidazopyridine H8), 7.90–7.92 (m, 3H, methylsulfonylphenyl H2 and H6, NH), 8.32 (d, 1H, J = 8.0 Hz, methylsulfonylphenyl H3 and H5); 13C NMR (DMSO-d6): δ ppm 18.86, 43.93, 55.61, 114.03, 114.15, 114.92, 115.70, 116.06, 122.48, 126.47, 127.36, 127.59, 136.72, 137.66, 139.20, 139.62, 141.93, 144.18, 152.50; LC-MS (ESI) m/z: 408 ([M + H]+, 100); Anal. Calcd. For C22H21N3O3S: C, 64.85; H, 5.19; N, 10.31. Found: C, 64.76; H, 5.22; N, 10.35.
Molecular modeling and docking studies
The docking studies between designed compounds and COX-2 isozyme were carried out by the AutoDock Vina program [43]. This procedure was accomplished after ligand and enzyme preparation. In brief, the 3D structure of murine COX-2 (ID: 6COX) was obtained from RCSB Protein Data Bank [44]. After eliminating the crystallized ligand and water molecules, polar hydrogens and Kollman charges were added to the protein. The 3D structures of two potent and selective derivatives were created and energetically minimized in HyperChem 8.0 software by the MM+ method. Then, the Gasteiger charges were added to ligands. Finally, the pdbqt files of ligands and the enzyme, which were used in docking created with AutoDock tools. Acquired configurations resulting from docking were searched to find suitable and efficient interactions with the COX-2 enzyme.
It was then validated by redocking the co-crystalized ligand, SC-558, under the same condition and superimposition on the co-crystallized ligand pose.
Biological assay
In vitro cyclooxygenase (COX) inhibition assays
This assay was performed using a COX fluorescent inhibitor screening assay kit (Cayman Chemical, MI, USA). The Cayman COX (ovine COX-1/human recombinant COX-2) inhibitor screening assay utilizes the peroxidase component of COXs. In this assay, the reaction between PGG2 and ADHP (10-acetyl-3,7-dihydroxyphenoxazine) produces the highly fluorescent compound resorufin. Resorufin fluorescence can be analyzed with a 530–540 nm excitation wavelength and an emission wavelength of 585–595 nm [45]. Consequently, higher inhibition of the COX enzyme leads to lower resorufin production, which means less fluorescence intensity.
In vivo evaluation of compound analgesic effects
Animals and reference drug
The analgesic effects of compounds were evaluated using a formalin test in rats [46]. Male Wistar rats (Pasteur Institute, Iran) weighing 110–150 g, were used. Rats were housed in a temperature-controlled condition (25 ± 2 °C) and 12 h light/dark cycle and free access to food and water except during the experiment. Animals were randomly divided into groups for each test compound (N = 6), and each rat was used only once during the experiments.
Formalin test
The basis of this pain assessment is the subcutaneous injection of formalin 5% into the paw and then monitoring of the animal’s pain-related behavior in test and control groups. Synthesized compounds or celecoxib (Sigma-Aldrich, Germany) were dissolved in DMSO and were administered by intraperitoneal (i.p.) injection (40 mg/kg, Volume of injection 1 ml/kg) 30 min before the test. The control group received DMSO (1 ml/kg) 30 min before the test. Formalin 5% (40 µl) was injected into the dorsal surface of the left hind paw, and the rats were placed individually in plexiglass chambers (30 × 30 × 30 cm) and continuously observed for 60 min. Pain-related behaviors were quantified according to the following numerical scale: 0 = normal weight-bearing on the injected paw, 1 = limping during locomotion or resting the paw lightly on the floor, 2 = elevation of the injected paw so that, at most, the nails touch the floor, and 3 = licking, biting or shaking the injected paw as described by Dubuisson and Dennis. The area under the curve (AUC) for pain score against the time plot was measured and compared between groups.
Statistical analysis of the data
Results were shown as mean and 95% confidence interval. Statistical analysis was done using Prism 6 (GraphPad Software Inc.). One-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison tests was used to compare AUCs of pain scores between groups. The p < 0.05 was regarded as statistically significant.
References
Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996;1299:125–40. https://doi.org/10.1016/0005-2760(95)00194-8.
Chandrasekharan NV, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA. 2002;99:13926–31. https://doi.org/10.1073/pnas.162468699.
Blobaum AL, Marnett LJ. Structural and functional basis of cyclooxygenase inhibition. J Med Chem. 2007;50:1425–41. https://doi.org/10.1021/jm0613166.
Giercksky KE. COX-2 inhibition and prevention of cancer. Best Pract Res Clin Gastroenterol. 2001;15:821–33. https://doi.org/10.1053/bega.2001.0237.
Wang D, DuBois RN. Cyclooxygenase-2: a potential target in breast cancer. Semin Oncol. 2004;31:64–73. https://doi.org/10.1053/j.seminoncol.2004.01.008.
Mahboubi Rabbani SMI, Zarghi A. Selective COX-2 inhibitors as anticancer agents: a patent review (2014-2018). Expert Opin Ther Pat. 2019;29:407–27. https://doi.org/10.1080/13543776.2019.1623880.
Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63:901–10. https://doi.org/10.1093/jnen/63.9.901.
Bhutani N, Moga S, Poswal P, Sharma B, Arora S, Singla S. COX-2 expression in carcinoma of the breast and surrounding non-neoplastic breast tissue. Archives of Breast Cancer. 2021:29–36. https://doi.org/10.32768/abc.20218129-36.
Prasher P, Sharma M, Gunupuru R. Targeting cyclooxygenase enzyme for the adjuvant COVID-19 therapy. Drug Dev Res. 2021;82:469–73. https://doi.org/10.1002/ddr.21794.
Dannhardt G, Kiefer W. Cyclooxygenase inhibitors—current status and future prospects. Eur J Med Chem. 2001;36:109–26. https://doi.org/10.1016/S0223-5234(01)01197-7.
Pu D, Yin L, Huang L, Qin C, Zhou Y, Wu Q, et al. Cyclooxygenase-2 inhibitor: a potential combination strategy with immunotherapy in cancer. Front Oncol. 2021;11:637504. https://doi.org/10.3389/fonc.2021.637504.
Li S, Jiang M, Wang L, Yu S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: recent advancement. Biomed Pharmacother. 2020;129:110389. https://doi.org/10.1016/j.biopha.2020.110389.
Zarghi A, Arfaei S. Selective COX-2 inhibitors: a review of their structure-activity relationships. Iran J Pharm Res. 2011;10:655–83. https://doi.org/10.22037/ijpr.2011.1047.
Mohsin N-U-A, Irfan M. Selective cyclooxygenase-2 inhibitors: a review of recent chemical scaffolds with promising anti-inflammatory and COX-2 inhibitory activities. Med Chem Res. 2020;29:809–30. https://doi.org/10.1007/s00044-020-02528-1.
Habeeb AG, Praveen Rao PN, Knaus EE. Design and synthesis of celecoxib and rofecoxib analogues as selective cyclooxygenase-2 (COX-2) inhibitors: replacement of sulfonamide and methylsulfonyl pharmacophores by an azido bioisostere. J Med Chem. 2001;44:3039–42. https://doi.org/10.1021/jm010153c.
Arefi H, Naderi N, Shemirani ABI, Kiani Falavarjani M, Azami Movahed M, Zarghi A. Design, synthesis, and biological evaluation of new 1,4-diarylazetidin-2-one derivatives (β-lactams) as selective cyclooxygenase-2 inhibitors. Arch Pharm. 2020;353:1900293. https://doi.org/10.1002/ardp.201900293.
Bekheit MS, Mohamed HA, Abdel-Wahab BF, Fouad MA. Design and synthesis of new 1,4,5-trisubstituted triazole-bearing benzenesulphonamide moiety as selective COX-2 inhibitors. Med Chem Res. 2021;30:1125–38. https://doi.org/10.1007/s00044-021-02716-7.
Ahmed EM, Kassab AE, El-Malah AA, Hassan MSA. Synthesis and biological evaluation of pyridazinone derivatives as selective COX-2 inhibitors and potential anti-inflammatory agents. Eur J Med Chem. 2019;171:25–37. https://doi.org/10.1016/j.ejmech.2019.03.036.
Zarghi A, Zebardast T, Hajighasemali F, Alipoor E, Daraie B, Hedayati M. Design and synthesis of new 1,3-benzdiazinan-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Arch Pharm. 2012;345:257–64. https://doi.org/10.1002/ardp.201100138.
Azami Movahed M, Daraei B, Shahosseini S, Esfahanizadeh M, Zarghi A. Design, synthesis, and biological evaluation of new pyrazino[1,2-a]benzimidazole derivatives as selective cyclooxygenase (COX-2) inhibitors. Arch Pharm. 2019;352:e1800265. https://doi.org/10.1002/ardp.201800265.
Abolhasani H, Zarghi A, Komeili Movahhed T, Abolhasani A, Daraei B, Dastmalchi S. Design, synthesis and biological evaluation of novel indanone containing spiroisoxazoline derivatives with selective COX-2 inhibition as anticancer agents. Bioorg Med Chem. 2021;32:115960. https://doi.org/10.1016/j.bmc.2020.115960.
Zarghi A, Ghodsi R, Azizi E, Daraie B, Hedayati M, Dadrass OG. Synthesis and biological evaluation of new 4-carboxyl quinoline derivatives as cyclooxygenase-2 inhibitors. Bioorg Med Chem. 2009;17:5312–7. https://doi.org/10.1016/j.bmc.2009.05.084.
Zarghi A, Ghodsi R. Design, synthesis, and biological evaluation of ketoprofen analogs as potent cyclooxygenase-2 inhibitors. Bioorg Med Chem. 2010;18:5855–60. https://doi.org/10.1016/j.bmc.2010.06.094.
Arfaie S, Zarghi A. Design, synthesis and biological evaluation of new (E)- and (Z)-1,2,3-triaryl-2-propen-1-ones as selective COX-2 inhibitors. Eur J Med Chem. 2010;45:4013–7. https://doi.org/10.1016/j.ejmech.2010.05.058.
Macarini AF, Sobrinho TUC, Rizzi GW, Corrêa R. Pyrazole–chalcone derivatives as selective COX-2 inhibitors: design, virtual screening, and in vitro analysis. Med Chem Res. 2019;28:1235–45. https://doi.org/10.1007/s00044-019-02368-8.
Farzaneh S, Shahhosseini S, Arefi H, Daraei B, Esfahanizadeh M, Zarghi A. Design, synthesis and biological evaluation of new 1,3-diphenyl-3- (phenylamino)propan-1-ones as selective cyclooxygenase (COX-2) inhibitors. Med Chem. 2018;14:652–9. https://doi.org/10.2174/1573406414666180525133221.
Soltani S, Abolhasani H, Zarghi A, Jouyban A. QSAR analysis of diaryl COX-2 inhibitors: comparison of feature selection and train-test data selection methods. Eur J Med Chem. 2010;45:2753–60. https://doi.org/10.1016/j.ejmech.2010.02.055.
Mirian M, Zarghi A, Sadeghi S, Tabaraki P, Tavallaee M, Dadrass O, et al. Synthesis and cytotoxic evaluation of some novel sulfonamidederivativesagainst a few human cancer cells. Iran J Pharm Res. 2011;10:741. https://doi.org/10.22037/ijpr.2011.980.
Bayanati M, Daraei B, Zarghi A, Daraei B. Design, synthesis, docking studies, enzyme inhibitory and antiplatelet aggregation activities of new 1,3-Diphenyl-3-(Phenylthio)Propan-1-One derivatives as selective COX-2 inhibitors. Anticancer Agents Med Chem. 2022. https://doi.org/10.2174/1871520622666220609111628.
Ahmaditaba MA, Shahosseini S, Daraei B, Zarghi A, Houshdar Tehrani MH. Design, synthesis, and biological evaluation of new peptide analogues as selective COX-2 inhibitors. Arch Pharm. 2017;350. https://doi.org/10.1002/ardp.201700158.
Singh P, Kaur S, Kaur J, Singh G, Bhatti R. Rational design of small peptides for optimal inhibition of cyclooxygenase-2: development of a highly effective anti-inflammatory agent. J Med Chem. 2016;59:3920–34. https://doi.org/10.1021/acs.jmedchem.6b00134.
Biava M, Battilocchio C, Poce G, Alfonso S, Consalvi S, Porretta GC, et al. Improving the solubility of a new class of antiinflammatory pharmacodynamic hybrids, that release nitric oxide and inhibit cycloxygenase-2 isoenzyme. Eur J Med Chem. 2012;58:287–98. https://doi.org/10.1016/j.ejmech.2012.10.014.
Ren S-Z, Wang Z-C, Zhu D, Zhu X-H, Shen F-Q, Wu S-Y, et al. Design, synthesis and biological evaluation of novel ferrocene-pyrazole derivatives containing nitric oxide donors as COX-2 inhibitors for cancer therapy. Eur J Med Chem. 2018;157:909–24. https://doi.org/10.1016/j.ejmech.2018.08.048.
Charlier C, Michaux C. Dual inhibition of cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem. 2003;38:645–59. https://doi.org/10.1016/S0223-5234(03)00115-6.
Li Z, Wang Z-C, Li X, Abbas M, Wu S-Y, Ren S-Z, et al. Design, synthesis and evaluation of novel diaryl-1,5-diazoles derivatives bearing morpholine as potent dual COX-2/5-LOX inhibitors and antitumor agents. Eur J Med Chem. 2019;169:168–84. https://doi.org/10.1016/j.ejmech.2019.03.008.
Uddin MJ, Rao PNP, Knaus EE. Design of acyclic triaryl olefins: a new class of potent and selective cyclooxygenase-2 (COX-2) inhibitors. Bioorg Med Chem Lett. 2004;14:1953–6. https://doi.org/10.1016/j.bmcl.2004.01.075.
Azami Movahed M, Daraei B, Zarghi A. Design synthesis and biological evaluation of new imidazo [1, 2-a] pyridine derivatives as selective cox-2 inhibitors. 2016;13:793–9. https://doi.org/10.1002/ardp.201800265.
Aakash D, Richa Kaur B, Ramanjot K, Sanjiv K, Upendra Kumar J, Harinder S, et al. Imidazo[1,2-a]pyridine Scaffold as prospective therapeutic agents. Curr Top Med Chem. 2017;17:238–50. https://doi.org/10.2174/1568026616666160530153233.
Tara LSK. Pyridines and imidazopyridines with medicinal significance. Curr Top Med Chem. 2016;16:3274–302. https://doi.org/10.2174/1568026616666160506145141.
Devi N, Singh D, Rawal RK, Bariwal J, Singh V. Medicinal attributes of imidazo [1, 2-a] pyridine derivatives: an update. Curr Top Med Chem. 2016;16:2963–94. https://doi.org/10.2174/1568026616666160506145539.
Congiu C, Cocco MT, Onnis V. Design, synthesis, and in vitro antitumor activity of new 1,4-diarylimidazole-2-ones and their 2-thione analogues. Bioorg Med Chem Lett. 2008;18:989–93. https://doi.org/10.1016/j.bmcl.2007.12.023.
Han X, Ma C, Wu Z, Huang G. Zinc iodide catalyzed synthesis of 3-aminoimidazo[1,2-a]pyridines from 2-aminopyridines and α-amino carbonyl compounds. Synthesis. 2016;48:351–6. https://doi.org/10.1055/s-0035-1560375.
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–91. https://doi.org/10.1002/jcc.21256.
Kurumbail R, Stallings W. Cyclooxygenase-2 (Prostaglandin Synthase-2) Complexed with a Selective Inhibitor, SC-558 IN I222 Space Group. 1997. https://www.rcsb.org/structure/6COX.
COX fluorescent inhibitor screening assay kit: cyman chemical. 2020; https://www.caymanchem.com/pdfs/700100.pdf.
Dubuisson D, Dennis SG. The formalin test: a quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4:161–74. https://doi.org/10.1016/0304-3959(77)90130-0.
Acknowledgements
The authors acknowledge the support from the Research Deputy of School of Pharmacy, Shahid Beheshti University of Medical Sciences, under grant number 20940.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The project was found to be in accordance with the ethical principles and the national norms and standards for conducting Medical Research in Iran (ethics code: IR.SBMU.PHARMACY.REC.1398.230).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Movahed, M.A., Abbasi, F.K., Rajabi, M. et al. Design, synthesis, and biological evaluation of new 2-(4-(methylsulfonyl)phenyl)-N-phenylimidazo[1,2-a]pyridin-3-amine as selective COX-2 inhibitors. Med Chem Res 32, 856–868 (2023). https://doi.org/10.1007/s00044-023-03041-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-023-03041-x