Abstract
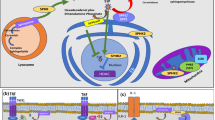
Inflammatory bowel disease (IBD) is an idiopathic intestinal inflammatory disease involving the ileum, rectum, and colon. Clinical manifestations include diarrhea, abdominal pain, and bloody stool. The disease includes ulcerative colitis (UC) and Crohn’s disease (CD). Biological therapies, including anti-TNF, anti-IL-12/23, and anti-integrins, improved the treatment of IBD, but they lack universal effectiveness and uniform immunogenicity. The advantage of small molecules over biological therapies includes tolerance of low immunogenicity, oral administration, and low manufacturing cost. Sphingosine 1-phosphate (S1P), derived from membrane sphingolipids, is a signaling molecule that is involved in immunological, cardiovascular, and neurological processes through interaction with sphingosine 1-phosphate receptors (S1PRs). S1P binds to S1PRs on the cell surface, activating multiple downstream signaling pathways such as AKT, Rac, Rho, ERK and PKC, causing a wide range of biological effects. S1PR is a G protein-coupled receptor with five subtypes: S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5. S1PR1 to 3 are expressed in various tissues, and S1PR4 is expressed in lymph nodes. S1PR5 is expressed in brain and skin. The S1PR agonists arise as new strategies for regulating downstream cytokine signaling in immune-mediated diseases. This article reviews the mechanisms of immune regulation by S1P/S1PRs, the pharmacokinetics of S1PR modulators (Fingolimod, Ozanimod, Etrasimod, Siponimod, among others), and the related clinical data.

S1PR subtypes and the medications that functionally modulate them



Similar content being viewed by others
Abbreviations
- S1PR:
-
Sphingosine-1-phosphate receptor
- EDG:
-
Endothelial differentiation gene
- Akt:
-
Serine/threonine kinase (protein kinase B)
- Rac:
-
Protein kinase B
- ERK:
-
Extracellular regulated protein kinases
- PLC:
-
Phospholipase C
- PKC:
-
Protein kinase C
- Rho:
-
Ras homolog gene family
- ROCK/ROK:
-
Rho activates Rho kinase
- CNS:
-
Central nervous system
- FDA:
-
Food and Drug Administration
- UC:
-
Ulcerative colitis
- CD:
-
Crohn’s disease
- IBD:
-
Inflammatory bowel disease
- CDAI:
-
Crohn’s disease activity index
- MS:
-
Multiple sclerosis
- JAK:
-
Janus kinase
- STAT:
-
Signal transducer and activator of transcription
- BBB:
-
Blood brain barrier
- BMS:
-
Bristol-Myers Squibb
- ARR:
-
Annual relapse rate
- OASIS:
-
Oxford Acute Severity of Illness Score
- mMCS:
-
Modified Mayo Clinic score
- MCS:
-
Mayo Clinic score
- BOLD:
-
Blood oxygen level dependent
- RRMS:
-
Relapsing-remitting multiple sclerosis
- SPMS:
-
Secondary progressive multiple sclerosis
- SLE:
-
Systemic lupus erythematosus
- AE:
-
Adverse effect
- TEAES:
-
Treatment-emergent AEs
- NF-κB:
-
Nuclear factor kappa-B
- IL:
-
Interleukin
- COX-2:
-
Cyclooxygenase-2
- RA:
-
Rheumatoid arthritis
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- Tmax:
-
Peak time
- Cmax:
-
Peak concentration
- AV block:
-
Atrioventricular block
References
Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P) physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–8. https://doi.org/10.1212/WNL.0b013e31820d5ec1.
Chun J, Giovannoni G, Hunter SF. Sphingosine 1-phosphate receptor modulator therapy for multiple sclerosis: differential downstream receptor signalling and clinical profile effects. Drugs. 2021;81:207–31. https://doi.org/10.1007/s40265-020-01431-8.
Park SJ, Im DS. Sphingosine 1-phosphate receptor modulators and drug discovery. Biomol Ther. 2017;25:80–90. https://doi.org/10.4062/biomolther.2016.160.
Waeber C, Walther T. Sphingosine-1-phosphate as a potential target for the treatment of myocardial infarction. Circ J. 2014;78:795–802. https://doi.org/10.1253/circj.CJ-14-0178.
Patmanathan SN, Wang W, Yap LF, Herr DR, Paterson IC. Mechanisms of sphingosine 1-phosphate receptor signalling in cancer. Cell Signal. 2017;34:66–75. https://doi.org/10.1016/j.cellsig.2017.03.002.
Intapad S. Sphingosine-1-phosphate signaling in blood pressure regulation. Am J Physiol Ren Physiol. 2019;317:E638–40. https://doi.org/10.1152/ajprenal.00572.2018.
Brunkhorst R, Vutukuri R, Pfeilschifter W. Fingolimod for the treatment of neurological diseases-state of play and future perspectives. Front Cell Neurosci. 2014;8. https://doi.org/10.3389/fncel.2014.00283.
Kono M, Proia RL. Imaging S1P1 activation in vivo. Exp Cell Res. 2015;333:178–82. https://doi.org/10.1016/j.yexcr.2014.11.023.
Kono M, Tucker AE, Tran J, Bergner JB, Turner EM, Proia RL. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J Clin Investig. 2014;124:2076–86. https://doi.org/10.1172/jci71194.
Blaho VA, Hla T. An update on the biology of sphingosine 1-phosphate receptors. J Lipid Res. 2014;55:1596–608. https://doi.org/10.1194/jlr.R046300.
Blankenbach KV, Schwalm S, Pfeilschifter J, zu Heringdorf DM. Sphingosine-1-phosphate receptor-2 antagonists: therapeutic potential and potential risks. Front Pharm. 2016;7:14. https://doi.org/10.3389/fphar.2016.00167.
Siehler S, Wang Y, Fan X, Windh RT, Manning DR. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem. 2001;276:48733–9. https://doi.org/10.1074/jbc.M011072200.
O’Sullivan MJ, Hirota N, Martin JG. Sphingosine 1-phosphate (S1P) induced interleukin-8 (IL8) release is mediated by S1P receptor 2 and nuclear factor kappa B in BEAS-2B cells. PLoS ONE. 2014;9:e95566. https://doi.org/10.1371/journal.pone.0095566.
Volzke A, Koch A, Heringdorf DMZ, Huwiler A, Pfeilschifter J. Sphingosine 1-phosphate (S1P) induces COX-2 expression and PGE(2) formation via S1P receptor 2 in renal mesangial cells. Biochim Biophys Acta Mol Cell Biol Lipids. 2014;1841:11–21. https://doi.org/10.1016/j.bbalip.2013.09.009.
Zhang GQ, Yang L, Kim GS, Ryan K, Lu SL, O’Donnell RK, et al. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood. 2013;122:443–55. https://doi.org/10.1182/blood-2012-11-467191.
Takabe K, Paugh SW, Milstien S, Spiegel S. “Inside-Out” signaling of sphingosine-1-phosphate: therapeutic targets. Pharm Rev. 2008;60:181–95. https://doi.org/10.1124/pr.107.07113.
Bravo GÁ, Cedeño RR, Casadevall MP, Ramió-Torrentà L. Sphingosine-1-phosphate (S1P) and S1P signaling pathway modulators, from current insights to future perspectives. Cells. 2022;11:2058. https://doi.org/10.3390/cells11132058.
Fryer RM, Muthukumarana A, Harrison PC, Mazurek SN, Chen RR, Harrington KE, et al. The clinically-tested S1P receptor agonists, FTY720 and BAF312, demonstrate subtype-specific bradycardia (S1P(1)) and hypertension (S1P(3)) in rat. PLoS ONE. 2012;7:9. https://doi.org/10.1371/journal.pone.0052985.
Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. https://doi.org/10.1016/j.pharmthera.2007.04.006.
Wang WG, Graeler MH, Goetzl EJ. Type 4 sphingosine 1-phosphate G protein-coupled receptor (S1P(4)) transduces S1P effects on T cell proliferation and cytokine secretion without signaling migration. Faseb J. 2005;19:1731. https://doi.org/10.1096/fj.05-3730fje.
Musumeci F, Greco C, Giacchello I, Fallacara AL, Ibrahim MM, Grossi G, et al. An update on JAK inhibitors. Curr Med Chem. 2019;26:1806–32. https://doi.org/10.2174/0929867325666180327093502.
Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O’Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Disco. 2017;16:843–62. https://doi.org/10.1038/nrd.2017.201.
Matsukawa A. STAT proteins in innate immunity during sepsis: lessons from gene knockout mice. Acta Med Okayama. 2007;61:239–45. https://doi.org/10.18926/amo/32897.
Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13:234–43. https://doi.org/10.1038/nrrheum.2017.23.
Xie WH, Xiao SY, Huang YR, Sun XY, Zhang ZL. Effect of tofacitinib on cardiovascular events and all-cause mortality in patients with immune-mediated inflammatory diseases: a systematic review and meta-analysis of randomized controlled trials. Ther Adv Musculoskelet Dis. 2019;11:18. https://doi.org/10.1177/1759720×19895492.
Venetsanopoulou AI, Voulgari PV, Drosos AA. Janus kinase versus TNF inhibitors: where we stand today in rheumatoid arthritis. Expert Rev Clin Immunol. 2022;18:485–93. https://doi.org/10.1080/1744666x.2022.2064275.
Atreya R, Billmeier U, Rath T, Neumann H, Neurath MF. Binding of membrane-bound TNF. In: Rogler G, Herfarth H, Hibi T, Nielsen OH, editors. Anti-tumor necrosis factor therapy in inflammatory bowel disease. Frontiers of Gastrointestinal Research. Basel: Karger; 2015. p. 62–72.
Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–46. https://doi.org/10.1038/nri1001.
Pugliese D, Privitera G, Fiorani M, Parisio L, Calvez V, Papa A, et al. Targeting IL12/23 in ulcerative colitis: update on the role of ustekinumab. Ther Adv Gastroenterol. 2022;15:17562848221102283. https://doi.org/10.1177/17562848221102283.
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, et al. The role of tumor necrosis factor alpha (TNF-alpha) in autoimmune disease and current TNF-alpha inhibitors in therapeutics. Int J Mol Sci. 2021;22:16. https://doi.org/10.3390/ijms22052719.
Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019;10:15. https://doi.org/10.1159/000509393.
Yarur AJ, Rubin DT. Therapeutic drug monitoring of anti-tumor necrosis factor agents in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2015;21:1709–18. https://doi.org/10.1097/mib.0000000000000380.
Olivera P, Danese S, Peyrin-Biroulet L. Next generation of small molecules in inflammatory bowel disease. Gut. 2017;66:199–209. https://doi.org/10.1136/gutjnl-2016-312912.
Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11:44–7. https://doi.org/10.1111/j.1524-4733.2007.00213.x.
Chun J, Kihara Y, Jonnalagadda D, Blaho VA. Fingolimod: lessons learned and new opportunities for treating multiple sclerosis and other disorders. In: Insel PA, editor. Annual review of pharmacology and toxicology, Vol 59. Palo Alto: Annual Reviews; 2019. p. 149–70.
Curro D, Pugliese D, Armuzzi A. Frontiers in drug research and development for inflammatory bowel disease. Front Pharm. 2017;8:19. https://doi.org/10.3389/fphar.2017.00400.
Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuropharmacol. 2010;33:91–101. https://doi.org/10.1097/WNF.0b013e3181cbf825.
Zecri FJ. From natural product to the first oral treatment for multiple sclerosis: the discovery of FTY720 (Gilenya (TM))? Curr Opin Chem Biol. 2016;32:60–6. https://doi.org/10.1016/j.cbpa.2016.04.014.
Kappos L, Antel J, Comi G, Montalban X, O’Connor P, Polman CH, et al. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–40. https://doi.org/10.1056/NEJMoa052643.
Cohen JA, Barkhof F, Comi G, Hartung HP, Khatri BO, Montalban X, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362:402–15. https://doi.org/10.1056/NEJMoa0907839.
Calabresi PA, Radue EW, Goodin D, Jeffery D, Rammohan KW, Reder AT, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13:545–56. https://doi.org/10.1016/s1474-4422(14)70049-3.
Cohen JA, Khatri B, Barkhof F, Comi G, Hartung H-P, Montalban X, et al. Long-term (up to 4.5 years) treatment with fingolimod in multiple sclerosis: results from the extension of the randomised TRANSFORMS study. J Neurol Neurosurg Psychiatry. 2016;87:468–75. https://doi.org/10.1136/jnnp-2015-310597.
Khatri B, Barkhof F, Comi G, Hartung HP, Kappos L, Montalban X, et al. Comparison of fingolimod with interferon beta-1a in relapsing-remitting multiple sclerosis: a randomised extension of the TRANSFORMS study. Lancet Neurol. 2011;10:520–9. https://doi.org/10.1016/s1474-4422(11)70099-0.
DiMarco JP, O’Connor P, Cohen JA, Reder AT, Zhang-Auberson L, Tang DJ, et al. First-dose effects of fingolimod: pooled safety data from three phase 3 studies. Mult Scler Relat Disord. 2014;3:629–38. https://doi.org/10.1016/j.msard.2014.05.005.
Ziemssen T, Albrecht H, Haas J, Klotz L, Lang M, Lassek C, et al. 36 months PANGAEA: a 5-year non-interventional study of safety, efficacy and pharmacoeconomic data for fingolimod patients in daily clinical practice. Mult Scler J. 2015;21:281–2. https://doi.org/10.1016/j.jval.2015.09.2894.
Bourdette D, Gilden D. Fingolimod and multiple sclerosis four cautionary tales. Neurology. 2012;79:1942–3. https://doi.org/10.1212/WNL.0b013e3182735edf.
Lindsey JW, Haden-Pinneri K, Memon NB, Buja LM. Sudden unexpected death on fingolimod. Mult Scler J. 2012;18:1507–8. https://doi.org/10.1177/1352458512438456.
Chaudhry BZ, Cohen JA, Conway DS. Sphingosine 1-phosphate receptor modulators for the treatment of multiple sclerosis. Neurotherapeutics. 2017;14:859–73. https://doi.org/10.1007/s13311-017-0565-4.
Rasche L, Paul F. Ozanimod for the treatment of relapsing remitting multiple sclerosis. Expert Opin Pharmacother. 2018;19:2073–86. https://doi.org/10.1080/14656566.2018.1540592.
Lassiter G, Melancon C, Rooney T, Murat AM, Kaye JS, Kaye AM, et al. Ozanimod to treat relapsing forms of multiple sclerosis: a comprehensive review of disease, drug efficacy and side effects. Neurol Int. 2020;12:89–108. https://doi.org/10.3390/neurolint12030016.
Squibb B-M. Food and Drug Administration approves Bristol Myers Squibbs ZEPOSIA® (Ozanimod), a new oral treatment for relapsing forms of multiple sclerosis. 2020. http://www.bms.com.
Scott FL, Clemons B, Brooks J, Brahmachary E, Powell R, Dedman H, et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P(1)) and receptor-5 (S1P(5)) agonist with autoimmune disease-modifying activity. Br J Pharmacol. 2016;173:1778–92. https://doi.org/10.1111/bph.13476.
Lamb YN. Ozanimod: first approval. Drugs. 2020;80:841–8. https://doi.org/10.1007/s40265-020-01319-7.
Tran JQ, Hartung JP, Olson AD, Mendzelevski B, Timony GA, Boehm MF, et al. Cardiac safety of ozanimod, a novel sphingosine-1-phosphate receptor modulator: results of a thorough QT/QTc study. Clin Pharm Drug Dev. 2018;7:263–76. https://doi.org/10.1002/cpdd.383.
Cohen JA, Comi G, SeImaj KW, Bar-Or A, Arnold DL, Steinman L, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (RADIANCE): a multicentre, randomised, 24-month, phase 3 trial. Lancet Neurol. 2019;18:1021–33. https://doi.org/10.1016/s1474-4422(19)30238-8.
Sandborn WJ, Feagan BG, Wolf DC, D’Haens G, Vermeire S, Hanauer SB, et al. Ozanimod induction and maintenance treatment for ulcerative colitis. N Engl J Med. 2016;374:1754–62. https://doi.org/10.1056/NEJMoa1513248.
Cohen JA, Arnold DL, Comi G, Bar-Or A, Gujrathi S, Hartung JP, et al. Safety and efficacy of the selective sphingosine 1-phosphate receptor modulator ozanimod in relapsing multiple sclerosis (RADIANCE): a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15:373–81. https://doi.org/10.1016/s1474-4422(16)00018-1.
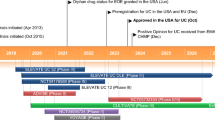
Hanzel J, Hulshoff MS, Grootjans J, D’Haens G. Emerging therapies for ulcerative colitis. Expert Rev Clin Immunol. 2022;18:513–24. https://doi.org/10.1080/1744666x.2022.2069562.
Gras J. Etrasimod arginine. Drug Future. 2020;45:165–73. https://doi.org/10.1358/dof.2020.45.3.3093451.
Peyrin-Biroulet L, Christopher R, Behan D, Lassen C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun Rev. 2017;16:495–503. https://doi.org/10.1016/j.autrev.2017.03.007.
Vermeire S, Chiorean M, Panes J, Peyrin-Biroulet L, Zhang JK, Sands BE, et al. Long-term safety and efficacy of etrasimod for ulcerative colitis: results from the open-label extension of the OASIS study. J Crohns Colitis. 2021;15:950–9. https://doi.org/10.1093/ecco-jcc/jjab016.
Sandborn WJ, Peyrin-Biroulet L, Zhang JK, Chiorean M, Vermeire S, Lee SD, et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158:550–61. https://doi.org/10.1053/j.gastro.2019.10.035.
Fathi I, Nishimura R, Imura T, Inagaki A, Kanai N, Ushiyama A, et al. KRP-203 is a desirable immunomodulator for islet allotransplantation. Transplantation. 2022;106:963–72. https://doi.org/10.1097/tp.0000000000003870.
Fujishiro J, Kudou S, Iwai S, Takahashi M, Hakamata Y, Kinoshita M, et al. Use of sphingosine-1-phosphate 1 receptor agonist, KRP-203, in combination with a subtherapeutic dose of cyclosporine a for rat renal transplantation. Transplantation. 2006;82:804–12. https://doi.org/10.1097/01.tp.0000232687.78242.cd.
Song J, Matsuda C, Kai Y, Nishida T, Nakajima K, Mizushima T, et al. A novel sphingosine 1-phosphate receptor agonist, 2-amino-2-propanediol hydrochloride (KRP-203), regulates chronic colitis in interleukin-10 gene-deficient mice. J Pharm Exp Ther. 2008;324:276–83. https://doi.org/10.1124/jpet.106.119172.
Shimizu H, Takahashi M, Kaneko T, Murakami T, Hakamata Y, Kudou S, et al. KRP-203, a novel synthetic immunosuppressant, prolongs graft survival and attenuates chronic rejection in rat skin and heart allografts. Circulation. 2005;111:222–9. https://doi.org/10.1161/01.Cir.0000152101.41037.Ab.
Radeke HH, Stein J, Van Assche G, Rogler G, Lakatos PL, Muellershausen F, et al. A multicentre, double-blind, placebo-controlled, parallel-group study to evaluate the efficacy, safety, and tolerability of the S1P receptor agonist KRP203 in patients with moderately active refractory ulcerative colitis. Inflamm Intest Dis. 2016:S285–S6. https://doi.org/10.1159/000509393.
Sugahara K, Maeda Y, Shimano K, Mogami A, Kataoka H, Ogawa K, et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br J Pharm. 2017;174:15–27. https://doi.org/10.1111/bph.13641.
Sugahara K, Maeda Y, Shimano K, Mogami A, Kataoka H, Ogawa K, et al. Amiselimod, a novel sphingosine 1-phosphate receptor-1 modulator, has potent therapeutic efficacy for autoimmune diseases, with low bradycardia risk. Br J Pharm. 2017;174:15–27. https://doi.org/10.1111/bph.13641.
Pérez-Jeldres T, Alvarez-Lobos M, Rivera-Nieves J. Targeting sphingosine-1-phosphate signaling in immune-mediated diseases: beyond multiple sclerosis. Drugs. 2021;81:985–1002. https://doi.org/10.1007/s40265-021-01528-8.
Kappos L, Arnold DL, Bar-Or A, Camm J, Derfuss T, Kieseier BC, et al. Safety and efficacy of amiselimod in relapsing multiple sclerosis (MOMENTUM): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2016;15:1148–59. https://doi.org/10.1016/s1474-4422(16)30192-2.
ClinicalTrials.gov. Amiselimod. 2022. https://clinicaltrials.gov/ct2/results?cond=Amiselimod&term=&cntry=&state=&city=&dist=.
Glaenzel U, Jin Y, Nufer R, Li WK, Schroer K, Adam-Stitah S, et al. Metabolism and disposition of siponimod, a novel selective S1P(1)/S1P(5) agonist, in healthy volunteers and in vitro identification of human cytochrome P450 enzymes involved in its oxidative metabolism. Drug Metab Dispos. 2018;46:1001–13. https://doi.org/10.1124/dmd.117.079574.
Spampinato SF, Merlo S, Sano Y, Kanda T, Sortino MA. Protective effect of the sphingosine-1 phosphate receptor agonist siponimod on disrupted blood brain barrier function. Biochemical Pharmacol. 2021;186:114465. https://doi.org/10.1016/j.bcp.2021.114465.
Gergely P, Nuesslein-Hildesheim B, Guerini D, Brinkmann V, Traebert M, Bruns C, et al. The selective sphingosine 1-phosphate receptor modulator BAF312 redirects lymphocyte distribution and has species-specific effects on heart rate. Br J Pharm. 2012;167:1035–47. https://doi.org/10.1111/j.1476-5381.2012.02061.x.
O’Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflamm. 2016;13:14. https://doi.org/10.1186/s12974-016-0494-x.
Kappos L, Li DKB, Stuve O, Hartung HP, Freedman MS, Hemmer B, et al. Safety and efficacy of siponimod (BAF312) in patients with relapsing-remitting multiple sclerosis dose-blinded, randomized extension of the phase 2 BOLD study. JAMA Neurol. 2016;73:1089–98. https://doi.org/10.1001/jamaneurol.2016.1451.
ClinicalTrials.gov. A multicenter, randomized, double-blind, parallel-group, placebo-controlled variable treatment duration study evaluating the efficacy and safety of Siponimod [BAF312] in patients with secondary progressive multiple sclerosis followed by extended treatment with open-label BAF312.2018. https://clinicaltrials.gov/ct2/show/NCT01665144?term=NCT01665144&rank=1.
Kappos L, Bar-Or A, Cree BAC, Fox RJ, Giovannoni G, Gold R, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391:1263–73. https://doi.org/10.1016/s0140-6736(18)30475-6.
Markham A. Ponesimod: first approval. Drugs. 2021;81:957–62. https://doi.org/10.1007/s40265-021-01523-z.
Baldin E, Lugaresi A. Ponesimod for the treatment of relapsing multiple sclerosis. Expert Opin Pharmacother. 2020;21:1955–64. https://doi.org/10.1080/14656566.2020.1799977.
Olsson T, Boster A, Fernandez O, Freedman MS, Pozzilli C, Bach D, et al. Oral ponesimod in relapsing-remitting multiple sclerosis: a randomised phase II trial. J Neurol Neurosurg Psychiatry. 2014;85:1198–208. https://doi.org/10.1136/jnnp-2013-307282.
ClinicalTrials.gov. Oral Ponesimod versus teriflunomide in relapsing multiple sclerosis (OPTIMUM) NCT02425644. 2015. https://clinicaltrials.gov/ct2/show/NCT02425644?term=NCT02425644&draw=2&rank=1.
Brossard P, Scherz M, Halabi A, Maatouk H, Krause A, Dingemanse J. Multiple-dose tolerability, pharmacokinetics, and pharmacodynamics of ponesimod, an S1P(1) receptor modulator: favorable impact of dose up-titration. J Clin Pharm. 2014;54:179–88. https://doi.org/10.1002/jcph.244.
ClinicalTrials.gov. A study of the safety and efficacy of ONO-4641 in patients with relapsing-remitting multiple sclerosis (DreaMS). 2010. https://clinicaltrials.gov/ct2/show/NCT01081782?term=Ceralifimod&draw=2&rank=1.
Krosser S, Wolna P, Fischer TZ, Boschert U, Stoltz R, Zhou MJ, et al. Effect of ceralifimod (ONO-4641) on lymphocytes and cardiac function: randomized, double-blind, placebo-controlled trial with an open-label fingolimod arm. J Clin Pharm. 2015;55:1051–60. https://doi.org/10.1002/jcph.513.
Xu JF, Gray F, Henderson A, Hicks K, Yang JS, Thompson P, et al. Safety, pharmacokinetics, pharmacodynamics, and bioavailability of GSK2018682, a sphingosine-1-phosphate receptor modulator, in healthy volunteers. Clin Pharm Drug Dev. 2014;3:170–8. https://doi.org/10.1002/cpdd.98.
Yu L, He L, Gan B, Ti R, Xiao Q, Hu H, et al. Structural insights into sphingosine-1-phosphate receptor activation. Proc Natl Acad Sci USA. 2022;119:e2117716119. https://doi.org/10.1073/pnas.2117716119.
ClinicalTrials.gov. Phase 1 study accessing the safety and tolerability of CBP-307.2014. https://clinicaltrials.gov/ct2/show/NCT02280434?term=CBP-307&draw=2&rank=4.
Author information
Authors and Affiliations
Contributions
Idea for the article: YW; Performed the literature search and data analysis: LX; Drafted the work: LX; Critically revised the work: PL.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, L., Lu, P. & Wang, Y. Sphingosine 1-phosphate receptor modulators for the treatment of inflammatory bowel disease and other immune-mediated diseases. Med Chem Res 31, 2074–2088 (2022). https://doi.org/10.1007/s00044-022-02961-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02961-4