Abstract
Kojic acid (KA) is a natural product containing 3-hydroxy-4-pyranone scaffold. It is produced by various aerobic microorganisms such as Aspergillus and Penicillium species. Since this bioactive compound can inhibit tyrosinase enzyme, it has been mainly used as a whitening agent in cosmetics. KA is a polyfunctional heterocycle providing a potential intermediate for the design and synthesis of new biologically active compounds. Numerous studies demonstrated that KA-derived compounds have significant antibacterial, antifungal, anticancer, anticonvulsant, anti-Alzheimer’s disease and metal chelating activities. In this review, we have described diverse therapeutic potential of 3-hydroxy-4-pyranones and related compounds as kojic acid analogs.

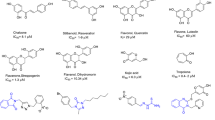
Modification of the natural product kojic acid has been considered for new lead generation with diverse biological activities.



































Similar content being viewed by others
References
Wilk W, Waldmann H, Kaiser M. Gamma-pyrone natural products–a privileged compound class provided by nature. Bioorg Med Chem. 2009;17:2304–9.
Chib S, Dogra A, Nandi U, Saran S. Consistent production of kojic acid from Aspergillus sojae SSC-3 isolated from rice husk. Mol Biol Rep. 2019;46:5995–6002.
Hashemi SM, Emami S. Kojic acid-derived tyrosinase inhibitors: synthesis and bioactivity. Pharm Biomed Res. 2015;1:1–17.
das Neves PAPFG, Lobato CC, Ferreira LR, Bragança VAN, Veiga AAS, Ordoñez ME, et al. Molecular modification approach on kojic acid derivatives as antioxidants related to ascorbic acid. J Mol Model. 2020;26:318.
Hosseinimehr SJ, Emami S, Zakaryaee V, Ahmadi A, Moslemi D. Radioprotective effects of kojic acid against mortality induced by gamma irradiation in mice. Saudi Med J. 2009;30:490–3.
Emami S, Hosseinimehr SJ, Taghdisi SM, Akhlaghpoor S. Kojic acid and its manganese and zinc complexes as potential radioprotective agents. Bioorg Med Chem Lett. 2007;17:45–48.
Kotani T, Ichimoto I, Tatsumi C, Fujita T. Structure-activity study of bacteriostatic kojic acid analogs. Agric Biol Chem. 1975;39:1311–7.
Kim JH, Chang PK, Chan KL, Faria NC, Mahoney N, Kim YK, et al. Enhancement of commercial antifungal agents by kojic acid. Int J Mol Sci. 2012;13:13867–80.
Rodrigues APD, Farias LHS, Carvalho ASC, Santos AS, Do Nascimento JLM, Silva EO. A novel function for kojic acid, a secondary metabolite from Aspergillus fungi, as antileishmanial agent. PLoS One. 2014;9:e91259.
Montazeri M, Emami S, Asgarian-Omran H, Azizi S, Sharif M, Sarvi S, et al. In vitro and in vivo evaluation of kojic acid against Toxoplasma gondii in experimental models of acute toxoplasmosis. Exp Parasitol. 2019;200:7–12.
Zirak M, Eftekhari-Sis B. Kojic acid in organic synthesis. Turk J Chem. 2015;39:439–96.
Saeedi M, Eslamifar M, Khezri K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed Pharmacother. 2019;110:582–93.
Selvin J, Maity D, Sajayan A, Kiran S. Revealing antibiotic resistance in therapeutic and dietary probiotic supplements. J Glob Antimicrob Re. 2020;22:202–5.
Wu Y, Shi YG, Zeng LY, Pan Y, Huang XY, Bian LQ, et al. Evaluation of antibacterial and anti-biofilm properties of kojic acid against five food-related bacteria and related subcellular mechanisms of bacterial inactivation. Food Sci Technol Int. 2019;25:3–15.
Liu X, Xia W, Jiang Q, Xu Y, Yu P. Synthesis, characterization, and antimicrobial activity of kojic acid grafted chitosan oligosaccharide. J Agric Food Chem. 2014;62:297–303.
Liu X, Jiang Q, Xia W. One-step procedure for enhancing the antibacterial and antioxidant properties of a polysaccharide polymer: Kojic acid grafted onto chitosan. Int J Biol Macromol. 2018;113:1125–33.
Aytemir MD, Hider RC, Erol DD, Özalp M, Ekizoğlu M. Synthesis of new antimicrobial agents; amide derivatives of pyranones and pyridinones. Turk J Chem. 2003;27:445–52.
Aytemir MD, Erol DD, Hider RC, Özalp M. Synthesis and evaluation of antimicrobial activity of new 3-hydroxy-6-methyl-4-oxo-4H-pyran-2-carboxamide derivatives. Turk J Chem. 2003;27:757–64.
Aytemir MD, Özçelik B. A study of cytotoxicity of novel chlorokojic acid derivatives with their antimicrobial and antiviral activities. Eur J Med Chem. 2010;45:4089–95.
Aytemir MD, Özçelik B. Synthesis and biological activities of new Mannich bases of chlorokojic acid derivatives. Med Chem Res. 2011;20:443–52.
Karakaya G, Aytemir MD, Özçelik B, Çalış Ü. Design, synthesis and in vivo/in vitro screening of novel chlorokojic acid derivatives. J Enzym Inhib Med Chem. 2013;28:627–38.
Aytemir MD, Özçelik B, Karakaya G. Evaluation of bioactivities of chlorokojic acid derivatives against dermatophytes couplet with cytotoxicity. Bioorg Med Chem Lett. 2013;23:3646–9.
Karakaya G, Türe A, Özdemir A, Özçelik B, Aytemir M. Synthesis and molecular modeling of some novel hydroxypyrone derivatives as antidermatophytic agents, J Heterocyclic Chem. 2022. https://doi.org/10.1002/jhet.4520.
Us D, Gürdal E, Berk B, Öktem S, Kocagöz T, Çağlayan B, et al. 4H-Pyran-4-one derivatives:; leading molecule for preparation of compounds with antimycobacterial potential. Turk J Chem. 2009;33:803–12.
Emami S, Ghafouri E, Faramarzi MA, Samadi N, Irannejad H, Foroumadi A. Mannich bases of 7-piperazinylquinolones and kojic acid derivatives: Synthesis, in vitro antibacterial activity and in silico study. Eur J Med Chem. 2013;68:185–91.
Reddy BS, Reddy MR, Madan CH, Kumar KP, Rao MS. Indium (III) chloride catalyzed three-component coupling reaction: A novel synthesis of 2-substituted aryl (indolyl) kojic acid derivatives as potent antifungal and antibacterial agents. Bioorg Med Chem Lett. 2010;20:7507–11.
Bingi C, Emmadi NR, Chennapuram M, Poornachandra Y, Kumar CG, Nanubolu JB, et al. One-pot catalyst free synthesis of novel kojic acid tagged 2-aryl/alkyl substituted-4H-chromenes and evaluation of their antimicrobial and anti-biofilm activities. Bioorg Med Chem Lett. 2015;25:1915–9.
Kim YG, Seo JH, Kwak JH, Shin KJ. Discovery of a potent enoyl-acyl carrier protein reductase (FabI) inhibitor suitable for antistaphylococcal agent. Bioorg Med Chem Lett. 2015;25:4481–6.
Flanagan ME, Brickner SJ, Lall M, Casavant J, Deschenes L, Finegan SM, et al. Preparation, Gram-negative antibacterial activity, and hydrolytic stability of novel siderophore-conjugated monocarbam diols. ACS Med Chem Lett. 2011;5:385–90.
Brown MF, Mitton-Fry MJ, Arcari JT, Barham R, Casavant J, Gerstenberger BS, et al. Pyridone-conjugated monobactam antibiotics with Gram-negative activity. J Med Chem. 2013;56:5541–52.
Tan L, Tao Y, Wang T, Zou F, Zhang S, Kou Q, et al. Discovery of novel pyridone-conjugated monosulfactams as potent and broad-spectrum antibiotics for multidrug-resistant gram-negative infections. J Med Chem. 2017;60:2669–84.
Xu B, Kong XL, Zhou T, Qiu DH, Chen YL, Liu MS, et al. Synthesis, iron (III)-binding affinity and in vitro evaluation of 3-hydroxypyridin-4-one hexadentate ligands as potential antimicrobial agents. Bioorg Med Chem Lett. 2011;21:6376–80.
Novais Â, Moniz T, Rebelo AR, Silva AM, Rangel M, Peixe L. New fluorescent rosamine chelator showing promising antibacterial activity against Gram-positive bacteria. Bioorg Chem. 2018;79:341–9.
Parrino B, Schillaci D, Carnevale I, Giovannetti E, Diana P, Cirrincione G, et al. Synthetic small molecules as anti-biofilm agents in the struggle against antibiotic resistance. Eur J Med Chem. 2019;161:154–78.
Çevik K, Ulusoy S. Inhibition of Pseudomonas aeruginosa biofilm formation by 2,2’-bipyridyl, lipoic, kojic and picolinic acids. Iran J Basic Med Sci. 2015;18:758–63.
Li YB, Liu J, Huang ZX, Yu JH, Xu XF, Sun PH, et al. Design, synthesis and biological evaluation of 2-substituted 3-hydroxy-6-methyl-4H-pyran-4-one derivatives as Pseudomonas aeruginosa biofilm inhibitors. Eur J Med Chem. 2018;158:753–66.
Telleria EL, Martins-da-Silva A, Tempone AJ, Traub-Csekö YM. Leishmania, microbiota and sand fly immunity. Parasitology. 2018;145:1336–53.
Mohammadbeigi A, Khazaei S, Heidari H, Asgarian A, Arsangjang S, Saghafipour A, et al. An investigation of the effects of environmental and ecologic factors on cutaneous leishmaniasis in the old world: a systematic review study. Rev Environ Health. 2020;36:117–28.
Emami S, Tavangar P, Keighobadi M. An overview of azoles targeting sterol 14α-demethylase for antileishmanial therapy. Eur J Med Chem. 2017;135:241–59.
Rodrigues APD, Farias LHS, Carvalho ASC, Santos AS, do Nascimento JLM, Silva EO. A novel function for kojic acid, a secondary metabolite from Aspergillus fungi, as antileishmanial agent. PloS one. 2014;9:e91259.
Sheikhmoradi V, Saberi S, Saghaei L, Pestehchian N, Fassihi A. Synthesis and antileishmanial activity of antimony (V) complexes of hydroxypyranone and hydroxypyridinone ligands. Res Pharm Sci. 2018;13:111–20.
Farhood B, Geraily G, Alizadeh A. Incidence and mortality of various cancers in Iran and compare to other countries: a review article. Iran J Public Health. 2018;47:309–16.
Mirzaei H, Shokrzadeh M, Modanloo M, Ziar A, Riazi GH, Emami S. New indole-based chalconoids as tubulin-targeting antiproliferative agents. Bioorg Chem. 2017;75:86–98.
Zhong L, Li Y, Xiong L, Wang W, Wu M, Yuan T, et al. Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct Target Ther. 2021;6:201.
Branosva J, Brtko J, Uher M, Novotny L. Antileukemic activity of 4-pyranone derivatives. Int J Biochem Cell Biol. 1995;27:701–6.
Fickova M, Pravdova E, Rondhal L, Uher M, Brtko J. In vitro antiproliferative and cytotoxic activities of novel kojic acid derivatives: 5-benzyloxy-2- selenocyanatomethyl- and 5-methoxy-2- selenocyanatomethyl-4-pyranone. J Appl Toxicol. 2008;28:554–9.
Yoo DS, Lee J, Choi SS, Rho HS, Cho DH, Shin WC, et al. A modulatory effect of novel kojic acid derivatives on cancer cell proliferation and macrophage activation. Pharmazie. 2010;65:261–6.
Chen Y-H, Lu P-J, Hulme C, Shaw AY. Synthesis of kojic acid-derived copper-chelating apoptosis inducing agents. Med Chem Res. 2013;22:995–1003.
Fu Y, Yang Y, Zhou S, Liu Y, Yuan Y, Li S, et al. Ciprofloxacin containing Mannich base and its copper complex induce antitumor activity via different mechanism of action. Int J Oncol. 2014;5:2092–2100.
Meier SM, Novak M, Kandioller W, Jakupec MA, Arion VB, Metzler-Nolte N, et al. Identification of the structural determinants for anticancer activity of a ruthenium arene peptide conjugate. Chem Eur J. 2013;19:9297–307.
Zhou Y, Tao P, Wang M, Xu P, Lu W, Lei P, et al. Development of novel human lactate dehydrogenase A inhibitors: High-throughput screening, synthesis, and biological evaluations. Eur J Med Chem. 2019;177:105–15.
Parthasarathy K, Praveen C, Balachandran C, Senthil kumar P, Ignacimuthu S, Perumal PT. Cu(OTf)2 catalyzed three component reaction: Efficient synthesis of spiro[indoline-3,4'-pyrano[3,2-b]pyran derivatives and their anticancer potency towards A549 human lung cancer cell lines. Bioorg Med Chem Lett. 2013;23:2708–13.
Shahrisa A, Esmati S, Miri R, Firuzi O, Edraki N, Nejati M. Cytotoxic activity assessment, QSAR and docking study of novel bis-carboxamide derivatives of 4-pyrones synthesized by Ugi four-component reaction. Eur J Med Chem. 2013;66:388–99.
Li Y-B, Hou W, Lin H, Sun P-H, Lin J, Chen W-M. Design, synthesis and biological evaluation of novel 5-hydroxy-2-methyl-4H-pyran-4-one derivatives as antiglioma agents. Med Chem Comm. 2018;9:471–6.
Beghi E. The epidemiology of epilepsy. Neuroepidemiology. 2020;54:185–91.
Atkinson JG, Rokach YGJ, Rooney CS, McFarlane CS, Rackham A, Share NN. Kojic amine–a novel gamma-aminobutyric acid analogue. J Med Chem. 1979;22:99–106.
Pelley KA, Vaught JL. An antinociceptive profile of kojic amine: an analogue of gamma-aminobutyric acid (GABA). Neuropharmacology. 1987;26:301–7.
Aytemir MD, Çaliş Ü, Özalp M. Synthesis and evaluation of anticonvulsant and antimicrobial activities of 3‐hydroxy‐6‐methyl‐2‐substituted 4H‐pyran‐4‐one derivatives. Arch Pharm. 2004;337:281–8.
Aytemir MD, Çalış Ü. Anticonvulsant and neurotoxicity evaluation of some novel kojic acids and allomaltol derivatives. Arch Pharm. 2010;343:173–81.
Giacobini E. Cholinesterases: new roles in brain function and in Alzheimer’s disease. Neurochem Res. 2003;28:515–22.
Gouras GK, Olsson TT, Hansson O. β-Amyloid peptides and amyloid plaques in Alzheimer’s disease. Neurotherapeutics. 2015;12:3–11.
Huang H-C, Jiang Z-F. Accumulated amyloid-β peptide and hyperphosphorylated tau protein: relationship and links in Alzheimer’s disease. J Alzheimers Dis. 2009;16:15–27.
Prakash A, Dhaliwal GK, Kumar P, Majeed ABA. Brain biometals and Alzheimer’s disease–boon or bane? Int J Neurosci. 2017;127:99–108.
Lu C, Guo Y, Yan J, Luo Z, Luo H-B, Yan M, et al. Design, synthesis, and evaluation of multitarget-directed a derivatives for the treatment of Alzheimer’s disease. J Med Chem. 2013;56:5843–59.
Hider R, Ma Y, Molina-Holgado F, Gaeta A, Roy S. Iron chelation as a potential therapy for neurodegenerative disease. Biochem Soc Trans. 2008;36:1304.
Tomic JL, Pensalfini A, Head E, Glabe CG. Soluble fibrillar oligomer levels are elevated in Alzheimer’s disease brain and correlate with cognitive dysfunction. Neurobiol Dis. 2009;35:352–8.
Xu P, Zhang M, Sheng R, Ma Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur J Med Chem. 2017;127:174–86.
Watkins PB, Zimmerman HJ, Knapp MJ, Gracon SI, Lewis KW. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. Jama. 1994;271:992–8.
Dgachi Y, Martin H, Malek R, Jun D, Janockova J, Sepsova V, et al. Synthesis and biological assessment of KojoTacrines as new agents for Alzheimer’s disease therapy. J Enzym Inhib Med Chem. 2019;34:163–70.
Arya A, Jokar S, Etemadfar P, Malekzadeh J, Jannesar R, Rohani M, et al. Comparison of deferoxamine, deferiprone and deferasirox iron-chelating agents in reducing serum ferritin levels in patients with thalassemia major. J Clin Care Skill. 2020;1:189–93.
Toso L, Crisponi G, Nurchi VM, Crespo-Alonso M, Lachowicz JI, Santos MA, et al. A family of hydroxypyrone ligands designed and synthesized as iron chelators. J Inorg Biochem. 2013;127:220–31.
Toso L, Crisponi G, Nurchi VM, Crespo-Alonso M, Lachowicz JI, Mansoori D, et al. Searching for new aluminium chelating agents: A family of hydroxypyrone ligands. J Inorg Biochem. 2014;130:112–21.
Ma Y, Luo W, Camplo M, Liu Z, Hider R. Novel iron-specific fluorescent probes. Bioorg Med Chem Lett. 2005;15:3450–2.
Nurchi VM, de M, Jaraquemada-Pelaez G, Crisponia G, Lachowicz JI, Cappai R, et al. A new tripodal kojic acid derivative for iron sequestration: Synthesis, protonation, complex formation studies with Fe3+, Al3+, Cu2+ and Zn2+, and in vivo bioassays. J Inorg Biochem. 2019;193:152–65.
Xie Y-Y, Lu Z, Kong X-L, Zhou T, Bansal S, Hider R. Systematic comparison of the mono-, dimethyl- and trimethyl 3- hydroxy-4(1H)-pyridones- Attempted optimization of the orally active iron chelator, deferiprone. Eur J Med Chem. 2016;115:132–40.
Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: A review. Vet World. 2018;11:627–35.
Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017;2:17023.
Lee M, Rho HS, Choi K. Anti-inflammatory effects of a P-coumaric acid and kojic acid derivative in LPS-stimulated RAW264.7 macrophage cells. Biotechnol Bioproc Engin. 2019;24:653–7.
Rho HS, Goh MI, Lee JK, Ahn SM, Yeon JH, Yoo DS, et al. Ester derivatives of kojic acid and polyphenols containing adamantane moiety with tyrosinase inhibitory and anti-inflammatory properties. Bull Korean Chem Soc. 2011;32:1411–14.
Zuo L, Prather ER, Stetskiv M, Garrison DE, Meade JR, Peace TI, et al. Inflammaging and oxidative stress in human diseases: from molecular mechanisms to novel treatments. Int J Mol Sci. 2019;20:4472.
Rho HS, Lee CS, Ahn SM, Hong YD, Shin SS, Park YH, et al. Studies on tyrosinase inhibitory and antioxidant activities of benzoic acid derivatives containing kojic acid moiety. Bull Korean Chem Soc. 2011;32:4411–4.
Chen YM, Li C, Zhang WJ, Shi Y, Wen ZJ, Chen QX, et al. Kinetic and computational molecular docking simulation study of novel kojic acid derivatives as anti-tyrosinase and antioxidant agents. J Enzym Inhib Med Chem. 2019;34:990–8.
Sharma DK, Pandey J, Tamrakar AK, Mukherjee D. Synthesis of heteroaryl/aryl kojic acid conjugates as stimulators of glucose uptake by GLUT4 translocation. Eur J Med Chem. 2014;85:727–736.
Acknowledgements
The authors are grateful to Mazandaran University of Medical Sciences for financial support (grant No. 5396).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emami, S., Ahmadi, R., Ahadi, H. et al. Diverse therapeutic potential of 3-hydroxy-4-pyranones and related compounds as kojic acid analogs. Med Chem Res 31, 1842–1861 (2022). https://doi.org/10.1007/s00044-022-02954-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02954-3