Abstract
A series of novel 10-Undecenoic acid-based triazole derivatives were designed and synthesized in the present study. 10-Undecenoic acid on treatment with propargyl bromide yielded the corresponding ester which was reacted with different aryl azides to obtain the novel triazoles. The synthesized compounds were characterized by NMR, IR, and mass spectral data. The synthesized lipidic triazole compounds were studied for the antibacterial and antifungal activities against Ralstonia solanacearum and Fusarium oxysporum, respectively. The antimicrobial assay results revealed that the synthesized compounds possessed a certain degree of antifungal and antibacterial activities on the tested organisms. Among all the compounds, it was found that compounds 4j (with iodo substituent) and 4q (tert-butyl substituent) displayed the highest antibacterial and antifungal activity respectively. The study revealed that novel lipidic triazole derivatives of 10-Undecenoic acid could be potential antimicrobial compounds. This is the first report on the design, synthesis, and antimicrobial assessment of 10-Undecenoic acid-based triazole against plant pathogenic microorganisms.

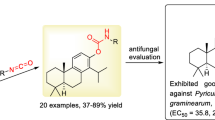
Graphical abstract


Similar content being viewed by others
Change history
04 January 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00044-022-03012-8
References
Tao, Industrial applications for plant oils and lipids. In: Bioprocessing for value-added products from renewable resources, Shang-Tian Yang, Elsevier, 611–27 (2004).
Ogunniyi DS. Castor oil: A vital industrial raw material. Bioresour Technol. 2006;97:1086–91. https://doi.org/10.1016/j.biortech.2005.03.028
Pabis S, Kula J. Synthesis and Bioactivity of (R)-ricinoleic acid derivatives: A Review. Curr Med Chem. 2016;23:4037–56. https://doi.org/10.2174/0929867323666160627104453
Van der SM, Stevens CV. Undecylenic acid: a valuable and physiologically active renewable building block from castor oil. ChemSusChem 2009;2:692–713. https://doi.org/10.1002/cssc.200900075
Sammaiah A, Kaki SS, Sai Manoj GNVT, Poornachandra Y, Kumar CG, Prasad RBN. Novel fatty acid esters of apocyninoxime exhibit antimicrobial and antioxidant activities. Eur J Lipid Sci Technol. 2015;117:692. https://doi.org/10.1002/ejlt.201400471
Desbois AP, Smith VJ. Antibacterial free fatty acids: activities, mechanisms of action and biotechnological potential. Appl Microbiol Biotechnol. 2010;85:1629–42. https://doi.org/10.1007/s00253-009-2355-3
Kanjilal S, Kaki SS Antimicrobial activities of fatty acids and their derivatives. In: The Royal Society of Chemistry, editor. AJ Domb, KK Reddy, S Farah: Antimicrobial materials for biomedical applications. 2019. p.457–80.
D’ Souza DM, Muller TJJ. Multi-component syntheses of heterocycles by transition-metal catalysis. ChemSoc Rev. 2007;36:1095–108. https://doi.org/10.1039/B608235C
Kharb R, Sharma PC, Yar MS. Pharmacological significance of triazole scaffold. J Enzym Inhib Med Chem. 2011;26:1–21. https://doi.org/10.3109/14756360903524304
Agalave SG, Maujan SR, Pore VS. Click chemistry: 1,2,3-Triazoles as pharcophores. Chem Asian J. 2011;6:2696–718. https://doi.org/10.1002/asia.201100432
Rajitha B, Jyothsna DP, Rajashaker B. Rational design, synthesis and antiproliferative evaluation of novel 1,4-benzoxazine-[1,2,3]triazole hybrids. Eur J Med Chem. 2015;89:138–46. https://doi.org/10.1016/j.ejmech.2014.10.051
Tangadanchu VKR, Sandhya GR, Bethala LAPD, Yedla P, Chityal GK. Isomannidemonoundecenoate-based 1,2,3-triazoles: Design, synthesis, and in vitro bioactive evaluation. J Heterocycl Chem. 2020;57:4312–21. https://doi.org/10.1002/jhet.4138
Jonathan AZ, Alberto N, Gary DS, Daniel KYS. Clickable Lipids: Azido and alkynyl fatty acids and triacylglycerols. J Am Oil Chem Soc. 2009;86:1115–21. https://doi.org/10.1007/s11746-009-1442-z
Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal. 2016;6:71–79. https://doi.org/10.1016/j.jpha.2015.11.005
Kolb HC, Sharpless KB. The growing impact of click chemistry on drug discovery. Drug Discov Today. 2003;8:1128–37. https://doi.org/10.1016/s1359-6446(03)02933-7
Antibacterial activity study of 1,2,4-triazole derivatives. Eur J Med Chem. 2019; 173:274–81. https://doi.org/10.1016/j.ejmech.2019.04.043.
Bilal L, Abdelghani D, Saida M, Abbes B. Synthesis and potential antimicrobial activity of novel α-aminophosphonates derivatives bearing substituted quinoline or quinolone and thiazole moieties. Med Chem Res. 2022;31:60–74. https://doi.org/10.1007/s00044-021-02815-5
Tang R, Jin L, Mou C, Yin J, Bai S, Hu D, et al. Synthesis, antifungal and antibacterial activity for novel amide derivatives containing a triazole moiety. Chem Cen J. 2013;7:30. https://doi.org/10.1186/1752-153X-7-30
Wu J, Kang S, Baoan S, Deyu, He M, Jin L, et al. Synthesis and antibacterial activity against Ralstoniasolanacearum for novel hydrazine derivatives containing a pyridine moiety. Chem Cen J. 2012;6:28. https://doi.org/10.1186/1752-153X-6-28
Duy TP, Thi MHV, Phuong T, Phuoc TH, Manh QN. Antimicrobial activity of some novel 2-(2-iodophenylimino)-5-arylidenethiazolidin- 4-one derivatives. Asian Biomed (Res Rev N.). 2017;11:405–12. https://doi.org/10.1515/abm-2018-0015
Marepu N, Yeturu S, Pal M. 1,2,3-Triazole fused with pyridine/pyrimidine as new template for antimicrobial agents: Regioselective synthesis and identification of potent N-heteroarenes. Bioorg Med Chem Lett. 2018;28:3302–6. https://doi.org/10.1016/j.bmcl.2018.09.021
Bew SP, Hiatt-Gipson GD. Synthesis of C-propargylicesters of N-protected amino acids and peptides. J Org Chem. 2010;75:3897–9. https://doi.org/10.1021/jo100537q
Kutonova KV, Trusova ME, Postnikov PS, Filimonov VD, Parello J. A simple and effective synthesis of aryl azides via arenediazoniumtosylates. Synthesis 2013;45:2706–10. https://doi.org/10.1055/s-0033-1339648
Sachin PS, Raghunath B, Green approach in click chemistry. In: Green chemistry. Hosam El-Din M Saleh and Martin Koller, IntechOpen. 2018;171–80. https://doi.org/10.5772/intechopen.72928.
Hudzicki, J. Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology. 2009.
Lee JY, Moon SS, Wang BK. Isolation and in vitro and in vivo activity against Phytophthoracapsici and Colletotrichumorbiculare of phenazine-1-carboxylic acid from Pseudomonas aeruginosa strain GC-B26. Pest Manag Sci. 2003;5:9872–882. https://doi.org/10.1002/ps.688
Adhikari M, Kharel N, Gaire L, Poudel R, Shrestha SM, Gaire SP, et al. In vitro evaluation of different chemicals against Rhizoctoniasolani by poisoned food technique. Field Crop. 2018;1:5–8. https://doi.org/10.5376/fc.2018.01.0002
Vincent JM. Distortion of fungal hyphae in the presence of certain inhibitors. Nature. 1947;159:850. https://doi.org/10.1038/159850b0
Acknowledgements
The authors also express sincere thanks to the Director, CSIR-IICT (Ms. No. IICT/Pubs./2022/126) for providing all the required facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gandhi, B., Greeshma, K., Ruvulapalli, D.P. et al. Design, synthesis, and antimicrobial evaluation of novel 10-Undecenoic acid-based lipidic triazoles. Med Chem Res 31, 1558–1570 (2022). https://doi.org/10.1007/s00044-022-02940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-022-02940-9