Abstract
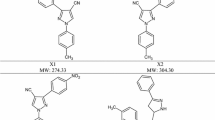
A series of substituted benzylidene-2-(4-phenylthiazol-2-yl) hydrazines (2a–q) have been synthesized, characterized and evaluated for their anti-inflammatory activity by carrageenin-induced hind paw edema (acute inflammation) and cotton pellet granuloma (chronic inflammation) methods in rats. In carrageenin-induced hind paw edema method, compounds 2a, 2b, 2c, 2d, 2h, 2k and 2p at a dose of 20 mg kg−1 body weight, p.o. showed excellent inhibitions (51.80–86.74 %) in between 1 and 4 h. Similarly, in cotton pellet granuloma method, compounds 2a, 2b, 2c, 2d, 2h, 2k and 2p at a dose of 20 mg kg−1 body weight, p.o. inhibited the granuloma formation (71.71–90.19 % inhibition) which was comparable to that of standard drug, ibuprofen (90.36 % inhibition of paw volume at 3 h and 94.02 % inhibition of granuloma formation). Structure activity relationship studies showed excellent activity of the compounds containing electron withdrawing group (fluoro, chloro, bromo or nitro) in phenyl ring at C2 and/or C4 position of thiazole ring.





Similar content being viewed by others
References
Bekhit AA, Fahmy HT, Rostom SA, Baraka AM (2003) Design and synthesis of some substituted 1H-pyrazolyl-thiazolo[4,5-d]pyrimidines as anti-inflammatory–antimicrobial agents. Eur J Med Chem 38:27–36
Bell FW, Cantrell AS, Hoegberg M, Jaskunas SR, Johansson NG, Jordan CL, Kinnick MD, Lind P, Morin JM Jr (1995) Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J Med Chem 38:4929–4936
Bharti SK, Nath G, Tilak R, Singh SK (2010) Synthesis, antibacterial and antifungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur J Med Chem 45:651–660
Bondock S, Khalifa W, Fadda AA (2007) Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem 42:948–954
Borisenko VE, Koll A, Kolmakov EE, Rjasnyi AG (2006) Hydrogen bonds of 2-aminothiazoles in intermolecular complexes (1:1 and 1:2) with proton acceptors in solutions. J Mol Struct 783:101–115
Buchanan JL, Bohacek RS, Luke GP, Hatada M, Lu X, Dalgarno DC, Narula SS, Yuan R, Holt DA (1999) Structure-based design and synthesis of a novel class of Src SH2 inhibitors. Bioorg Med Chem Letts 9:2353–2358
Crunkhorn P, Meacock SC (1971) Mediators of the inflammation induced in the rat paw by carrageenin. Br J Pharmacol 42:392–402
da Silva CM, da Silva DL, Modolo LV, Alves RB, de Resende MA, Martins CV, de Fatima A (2011) Schiff bases: a short review of their antimicrobial activities. J Adv Res 2:1–8
de Menezes MR, Catanzaro-Guimaraes SA (1985) Determination of anti-inflammatory and antimitotic activities of non-steroid anti-inflammatory drugs ibuprofen, diclofenac sodium and fentiazac. Cell Mol Biol 31(6):455–461
Di Rosa M (1972) Biological properties of carrageenan. J Pharm Pharmacol 24:89–102
El Kazzouli S, Berteina-Raboin S, Mouaddib A, Guillaumet G (2002) Solid support synthesis of 2,4-disubstituted thiazoles and aminothiazoles. Tetrahedron Lett 43:3193–3196
El-Subbagh HI, Al-Obaid AM (1996) 2,4-disubstitued thiazoles II. A novel class of antitumor agents, synthesis and biological evaluation. Eur J Med Chem 31:1017–1021
Fink BE, Mortensen DS, Stauffer SR, Aron ZD, Katzenellenbogen JA (1999) Novel structural templates for estrogen-receptor ligands and prospects for combinatorial synthesis of estrogens. Chem Biol 6:205–219
Gu XH, Wan XZ, Jiang B (1999) Syntheses and biological activities of bis(3-indolyl)thiazoles, analogues of marine bis(indole)alkaloid nortopsentins. Bioorg Med Chem Letts 9:569–572
Hallinan EA, Hagen TJ, Tsymbalov S, Stapelfeld A, Savage MA (2001) 2,4-Disubstituted oxazoles and thiazoles as latent pharmacophores for diacylhydrazine of SC-51089, a potent PGE2 antagonist. Bioorg Med Chem 9:1–6
Holla BS, Malini KV, Rao BS, Sarojini BK, Kumari NS (2003) Synthesis of some new 2,4-disubstituted thiazoles as possible antimicrobial and anti-inflammatory agents. Eur J Med Chem 38:313–318
Jiang B, Gu XH (2000) Syntheses and cytotoxicity evaluation of bis(indolyl)thiazole, bis(indolyl)pyrazinone and bis(indolyl)pyrazine: analogues of cytotoxic marine bis(indole) alkaloid. Bioorg Med Chem 8:363–371
Karegoudar P, Karthikeyan MS, Prasad DJ, Mahalinga M, Holla BS, Kumari NS (2008) Synthesis of some novel 2,4-disubstituted thiazoles as possible antimicrobial agents. Eur J Med Chem 43:261–267
Kaspady M, Narayanaswamy VK, Raju M, Rao GK (2009) Synthesis, antibacterial activity of 2,4-disubstituted oxazoles and thiazoles as bioisosteres. Lett Drug Design Discovery 6:21–28
Kolb J, Beck B, Almstetter M, Heck S, Herdtweck E, Domling A (2003) New MCRs: the first 4-component reaction leading to 2,4-disubstituted thiazoles. Mol Divers 6:297–313
Kouadio F, Kanko C, Juge M (2000) Analgesic and anti-inflammatory activities of an extract from Parkia biglobosa used in traditional medicine in the Ivory Coast. Phytother Res 14:635–637
Kumar Y, Green R, Borysko KZ, Wise DS, Wotring LL, Townsend LB (1993a) Synthesis of 2,4-disubstituted thiazoles and selenazoles as potential antitumor and antifilarial agents: 1. Methyl 4-(Isothiocyanatomethyl)thiazole-2-carbamates,-selenazole-2-carbamates, and related derivatives. J Med Chem 36:3843–3848
Kumar Y, Green R, Wise DS, Wotring LL, Townsend LB (1993b) Synthesis of 2,4-disubstituted thiazoles and selenazoles as potential antifilarial and antitumor agents. 2. 2-arylamido and 2-alkylamido derivatives of 2-amino-4-(isothiocyanatomethyl)thiazole and 2-amino-4-(isothiocyanatomethyl)selenazole. J Med Chem 36:3849–3852
Lednicer D, Mitscher LA, George GI (1990) Organic chemistry of drug synthesis, vol 4. Wiley, New York, p 95
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25
Loncle C, Brunel J, Vidal N, Dherbomez M, Letourneux Y (2004) Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 39:1067–1071
Martelli AE (1977) Inflammation and anti-inflammatories. Spectrum publications, New York, p 177
Metzger JV (1984) Comprehensive heterocyclic chemistry I, vol 6. Pergamon, New York, p 328
Molina J (1985) Fentiazac in acute gouty arthritis. Clin Ther 7:327–333
Mossa JS, Rafatullah S, Galal AM, Al-Yahya MA (1995) Pharmacological studies of Rhus retinorrharaI. Int J Pharmacognosy 33:242–246
Muri EMF, Mishra H, Stein SM, Williamson JS (2004) Molecular modeling, synthesis and biological evaluation of heterocyclic hydroxamic acids designed as Helicobacter pylori urease inhibitors. Lett Drug Design Discov 1:30–34
Odds FC, Brown AJ, Gow NA (2003) Antifungal agents: mechanisms of action. Trends Microbiol 11:272–279
Patt WC, Hamilton HW, Taylor MD, Ryan MJ, Taylor DG Jr, Connolly CJ, Doherty AM, Klutchko SR, Sircar I (1992) Structure-activity relationships of a series of 2-amino-4-thiazole-containing renin inhibitors. J Med Chem 35:2562–2572
Potewar TM, Ingale SA, Srinivasan KV (2007) Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: a practical approach towards the synthesis of fanetizole. Tetrahedron 63:11066–11069
Russo F, Guccione S, Romeo G, Barretta GU, Pussi S, Cutuli V (1993) Pyrazolothiazolopyrimidine derivatives as a novel class of anti-inflammatory or antinociceptive agents: synthesis, structural characterization and pharmacological evaluation. Eur J Med Chem 28:363–376
Sharma PK, Sawhney SN (1997) Potent antiinflammatory 3-thiazole-4(5)-acetic acids of 1,2-benzisothiazole. Bioorg Med Chem Lett 7:2427–2430
Siddiqui N, Ahsan W (2010) Triazole incorporated thiazoles as a new class of anticonvulsants: design, synthesis and in vivo screening. Eur J Med Chem 45(4):1536–1543
Spector WG (1969) The granulomatous inflammatory exudates. Int Rev Exp Pathol 8:1–55
Swingle KF, Shideman FE (1972) Phases of the inflammatory response to subcutaneous implantation of a cotton pellet and their modification by certain antiinflammatory agents. J Pharmacol Exp Ther 183:226–234
van Muijlwijk-Koezen JE, Timmerman H, Vollinga RC, von Drabbe Kunzel JF, de Groote M, Visser S, IJzerman AP (2001) Thiazole and thiadiazole analogues as a novel class of adenosine receptor antagonists. J Med Chem 44:749–762
Wilson KJ, Illig CR, Subasinghe N, Hoffman JB, Rudolph MJ, Soll R, Molloy CJ, Bone R, Green D, Randall T, Zhang M, Lewandowski FA, Zhou Z, Sharp C, Maguire D, Grasberger B, DesJarlais RL, Spurlino J (2001) Synthesis of thiophene-2-carboxamidines containing 2-amino-thiazoles and their biological evaluation as urokinase inhibitors. Bioorg Med Chem Letts 11:915–918
Winter CA, Risley EA, Nuss CW (1962) Carrageenan-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med 111:544–547
Zimmerman M (1983) Ethical guidelines for investigation of experimental pain in conscious animal. Pain 16:109–110
Acknowledgments
The authors are grateful to the Head, Department of Chemistry, Faculty of Science, Banaras Hindu University (BHU), Varanasi, India for 1H and 13C NMR spectrometry, Indian Institute of Chemical Technology (IICT), Hyderabad for mass spectrometry. S.K. Bharti is grateful to University Grants Commission (UGC), New Delhi for the award of senior research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bharti, S.K., Singh, S.K. Design, synthesis and biological evaluation of some novel benzylidene-2-(4-phenylthiazol-2-yl) hydrazines as potential anti-inflammatory agents. Med Chem Res 23, 1004–1015 (2014). https://doi.org/10.1007/s00044-013-0708-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0708-z