Abstract
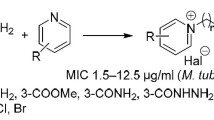
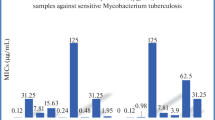
A series of phenothiazine clubbed pyrazolo[3,4-d]pyrimidines have been synthesized by using the Biginelli multi-component cyclocondensation reaction and their ability to inhibit growth of Mycobacterium tuberculosis in vitro have been determined. The results show that compounds 4b, 4d, and 4f exhibited excellent anti-tubercular activity with percentage inhibition of 93, 91, and 96, respectively, at a minimum inhibitory concentration (MIC) of <6.25 μg/ml, whereas compounds 4a, 4c, 4e, 4g, and 4h exhibited moderate to good anti-tubercular activity with percentage inhibition of 75, 68, 74, 54, and 63, respectively at a MIC of >6.25 μg/ml.

Similar content being viewed by others
References
Albery WJ, Foulds AW, Hall KJ, Hillman AR, Edgell RG, Orchard AF (1979) Thionine coated electrode for photogalvanic cells. Nature 282:793–797
Brun A, Harriman M, Heitz V, Sauvage JP (1991) Charge transfer across oblique bisporphyrins: two-center photoactive molecules. J Am Chem Soc 113:8657–8663
Collins L, Franzblau SG (1997) Microplate Alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob Agents Chemother 41:1004–1009
Davies LP, Chow SC, Skerritt JH, Brown DJ, Johnston GAR (1984) Pyrazolo[3, 4-d]pyrimidines as adenosine antagonists. Life Sci 34:2117–2128
Duesing R, Tapolsky G, Meyer T (1990) Long-range, light-induced redox separation across a ligand bridge. J Am Chem Soc 112:5378–5379
Filler R (1974) Fluorinated compounds of medicinal interest. Chem Technol 4:752–757
Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Quenzer VK, Freguson RM, Gilman RH (1998) Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar blue assay. J Clin Microb 36:362–366
Ghorab MM, Ismail ZH, Abdel-Gawad SM, Abdel Aziem A (2004) Antimicrobial activity of amino acid, imidazole, and sulfonamide derivatives of pyrazolo[3, 4-d]pyrimidine. Heteroat Chem 15:57–62
Ibrahim Abdou M, Saleh AM, Zohdi HF (2004) Synthesis and antitumor activity of 5-trifluoromethyl-2,4-dihydropyrazol-3-one nucleosides. Molecules 9:109–116
Julino M, Stevens MFG (1998) Synthesis of 7H-pyrido[4,3,2,-Kl]acridines with exploitable functionality in the pyridine ring. J Chem Soc Perkin Trans 1: Org Bio-Org Chem 10:1677–1684
Knorr A, Daub J (1995) Luminescence stimulated by electron transfer: fluorescent donor/acceptor-substituted stilbenes containing pyrenoid and heteroaromatic subunits. Angew Chem Int Ed Engl 34:2664–2666
Mcintyre R, Gerischer H (1984) Electron transfer reactions at n-gallium phosphide (100) and (111) in acetonitrile solutions facilitated by cation adsorption. Ber Bunsen Ges Phys Chem 88:963–969
Mietzsch F (1954) The development of antihistaminics and central damping agents. Angew Chem 66:363–371
Moutet JC, Reverdy J (1983) Photochemistry of cation radicals in solution. Photoinduced electron-transfer reaction: oxidation of 1, 1-diarylethylenes. Nouv J Chim 7:105–111
Nishiwaki E, Nakagawa H, Takasaki M, Matsumoto T, Sakurai H, Shibuya M (1990) Synthesis of oligo-N-methylpyrrolecarboxamide derivatives and their photochemical DNA-cleaving activities. Heterocycles 31:1763–1767
Okafor OC (1977) Studies in the heterocyclic series. XII. The chemistry and applications of aza and thia analogs of phenoxazine and related compounds. Heterocycles 7:391–427
Scior T, Meneses Morales I, Garce′s Eisele SJ, Domeyer D, Laufer S (2002) Antitubercular isoniazid and drug resistance of Mycobacterium tuberculosis—a review. Arch Pharm 335:511–525
Spreitzer H, Daub J (1996) Multi-mode switching based on dihydroazulene/vinylheptafulvene photochromism: synergism of photochromism and redox switching in heteroaryl-functionalized systems. Chem Eur J 2:1150–1158
Suling WJ, Seitz LE, Pathak V, Westbrook L, Barrow EW, Zywno-van-ginkel S, Renolds RC, Piper JR, Barrow WW (2000) Antimycobacterial activities of 2,4-diamino-5-deazapteridine derivatives and effects on mycobacterial dihydrofolate reductase. Antimicrob Agents Chemother 44:2784–2793
Tripathi RP, Tewari N, Dwivedi N, Tiwari VK (2005) Fighting tuberculosis: an old disease with new challenges. Med Res Rev 25:93–131
Trivedi AR, Siddiqui AB, Shah VH (2008a) Design, synthesis, characterization and antitubercular activity of some 2-heterocycle-substituted phenothiazines. Arkivoc 2:210–217
Trivedi A, Dodiya D, Surani J, Mathukia H, Ravat N, Shah V (2008b) Facile one-pot synthesis and antimycobacterial evaluation of pyrazolo[3,4-d]pyrimidines. Arch Pharm 341:435–439
Yajko DM, Madej JJ, Lacaster MV, Sanders CA, Cawthon VL, Gee B, Babst A, Keith Hardley W (1995) Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microb 33:2324–2327
Acknowledgments
This study was funded by the CSIR through Grant No. 01(02348)/09/EMR-II. The authors gratefuly acknowledge the CSIR for its financial support. The authors are indebted to the Tuberculosis Antimicrobial Acquisition and Coordinating Facility (TAACF/USA) for biological tests. Amit Trivedi is thankful to CSIR, New Delhi for fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trivedi, A.R., Dholariya, B.H., Vakhariya, C.P. et al. Synthesis and anti-tubercular evaluation of some novel pyrazolo[3,4-d]pyrimidine derivatives. Med Chem Res 21, 1887–1891 (2012). https://doi.org/10.1007/s00044-011-9712-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9712-3