Abstract
Skeletal disorders are problematic aspects for the aquaculture industry as skeletal deformities, which affect most species of farmed fish, increase production costs and affect fish welfare. Following recent findings that show the presence of osteoactive compounds in marine organisms, we evaluated the osteogenic and mineralogenic potential of commercially available microalgae strains Skeletonema costatum and Tetraselmis striata CTP4 in several fish systems. Ethanolic extracts increased extracellular matrix mineralization in gilthead seabream (Sparus aurata) bone-derived cell cultures and promoted osteoblastic differentiation in zebrafish (Danio rerio) larvae. Long-term dietary exposure to both extracts increased bone mineralization in zebrafish and upregulated the expression of genes involved in bone formation (sp7, col1a1a, oc1, and oc2), bone remodeling (acp5a), and antioxidant defenses (cat, sod1). Extracts also improved the skeletal status of zebrafish juveniles by reducing the incidence of skeletal anomalies. Our results indicate that both strains of microalgae contain osteogenic and mineralogenic compounds, and that ethanolic extracts have the potential for an application in the aquaculture sector as dietary supplements to support fish bone health. Future studies should also identify osteoactive compounds and establish whether they can be used in human health to broaden the therapeutic options for bone erosive disorders such as osteoporosis.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Osteogenic compounds that can stimulate bone formation and mineralization have received increasing attention by researchers in the field of aquaculture due to the existence of skeletal disorders that significantly impact the value and welfare of farmed fish. High incidences of skeletal anomalies are observed in virtually all species of reared fish. These anomalies alter fish swimming behavior and feeding abilities, thus diminish welfare and survival, but also negatively impact on customer perception and sales, thus limit profits for the industry [1, 2]. The supplementation of fish diets with ingredients that support a healthy skeletal development is seen as a suitable strategy to reduce the incidence of skeletal anomalies in aquaculture species [2]. Natural compounds with the capacity to induce bone formation and mineralization (i.e., osteoanabolic compounds) have been uncovered in a variety of organisms, including marine organisms [3,4,5,6,7]; these compounds have the potential to be the basis of new dietary supplements to improve the skeletal health of farmed fish [8].
In this scenario, marine microalgae have gained momentum in applications related to the blue economy and sustainable growth [9], ranging from clean energy production [10], wastewater treatment [11], animal feeds [12, 13] to human nutrition and medicine [14, 15]. They are optimal sources of essential nutrients [16], and being able to produce a plethora of bioactive molecules [17], they are expected to fuel pharmaceutical development in the upcoming years [18]. The increasing interest of academia and industries for microalgae has stimulated the development of methods for the large-scale production of biomass and genetic engineering tools have recently been successfully applied to microalgae, allowing for the development of transgenic lines optimized for the synthesis of specific compounds, shaping the idea of microalgae as full-fledge bioreactors for the production of biomolecules [19,20,21].
Despite a growing interest for microalgae-derived bioactives, very few studies have explored microalgae as a source of osteogenic and mineralogenic compounds, possibly because of the perceived lack of suitable systems to assess these bioactivities. In this regard, small teleosts such as the zebrafish (Danio rerio) presents several technical advantages over classical mammalian models, including lower maintenance costs, small size, short life cycle, high fecundity, easier genetic manipulation, and translucent embryonic stages [22,23,24,25,26,27]. For the same advantages provided to biomedical research and in virtue of its evolutionary resemblance with teleosts of commercial interest, zebrafish is also becoming a popular model for aquaculture research, being successfully implemented for research in fish nutrition and immunology [28, 29]. Several fish-based in vitro and in vivo systems have been recently described [30,31,32,33,34,35] and successfully used to assess the presence of osteoactive compounds found in marine invertebrates, seaweeds, and halophyte plants [3,4,5,6,7]. In the present work, extracts from two commercially available species of marine microalgae—Skeletonema costatum and Tetraselmis striata CTP4—were prepared through ethanolic maceration and evaluated for the presence of osteogenic and mineralogenic compounds using a diverse set of screening systems.
Materials and methods
Preparation of ethanolic extracts
Freeze-dried biomass of Skeletonema costatum (Necton S.A., Olhão, Portugal) and Tetraselmis striata CTP4 (ALLMICROALGAE—Natural Products S.A., Pataias, Portugal) was macerated with 96% ethanol (Merck, Darmstadt, Germany) at a ratio of 1 g of biomass for 40 mL of solvent, under gentle agitation at 24 °C for 18 h. Macerates were centrifuged for 5 min at 1000×g and supernatants were collected. Pellets were washed twice with 96% ethanol and supernatants were pooled and then vacuum filtered sequentially through 0.45 µm and 0.22 µm nylon membranes (Labbox Labware S.L. Barcelona, Spain). Filtrates were kept at – 20 °C until concentrated using a rotatory evaporator RV 10 digital (IKA-Werke GmbH & Co, Staufen im Breisgau, Germany) at 40 °C. Extracts were evaporated until a dense paste was formed. Extraction yields were determined from 2 mL of each concentrated solution placed under a gentle flow of 99.8% nitrogen until complete evaporation. The yield of the extraction process was 37.9% for S. costatum and 19.6% for T. striata CTP4 (Fig. 1A).
Cell culture and extracellular matrix mineralization
A previously established gilthead seabream (Sparus aurata) line of osteochondroprogenitor cells (VSa13; Cellosaurus accession number CVCL_S952) [30] was maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, 1% L-glutamine, and 0.2% fungizone (all from GIBCO, ThermoFisher Scientific, Waltham, USA) at 33 °C in a 10% CO2-humidified atmosphere [30, 31]. Pre-confluent cell cultures were sub-cultured 1:4 twice a week using trypsin–EDTA solution (0.2% trypsin from GIBCO, 1.1 mM EDTA, pH 7.4). Mineralization assays were conducted in 48-well plates seeded at a density of 1.25 × 104 cells per well (1.56 × 104 cells/cm2) and incubated until culture reached confluency. Extracellular matrix (ECM) mineralization was induced by supplementing culture medium with L-ascorbic acid (50 μg/mL), β-glycerophosphate (10 mM) and calcium chloride (4 mM). Mineralizing cell cultures were treated with ethanolic extracts from Skeletonema costatum or Tetraselmis striata CTP4 (thereafter referred to as SKLT and CTP4) at different concentrations, or with their vehicle (0.1% ethanol), thereafter designed as the control group (CTRL). Cells cultured in non-supplemented medium (i.e., without mineralogenic cocktail and extracts) were also used as a negative control (Min−). After 17 days of culture, mineral deposition was assessed through alizarin red S (AR-S, Sigma-Aldrich, St. Louis, USA) staining and quantified by spectrophotometry as previously described [30].
Zebrafish maintenance
Zebrafish wild-type (AB) and transgenic lines Tg(Hsa.RUNX2-Mmu.Fos:EGFP)zf259, Tg(Ola.Sp7:mCherry-Eco.NfsB)pd46 and Tg(Ola.osteocalcin:EGFP)hu4008, hereafter referred to as Tg(runx2:EGFP), Tg(sp7:mCherry) and Tg(oc:EGFP) [36, 37], were maintained in a ZebTEC water recirculating system (Tecniplast, Buguggiate, Italy) at the aquatic animal facilities of the Centre of Marine Sciences (CCMAR, Faro, Portugal). Eggs were obtained following an in-house breeding program using sexually mature adult zebrafish. Viable fertilized eggs were transferred into plastic 1 L tanks and maintained for 3 days in reverse osmosis-treated water supplemented with salts (Instant Ocean, Blacksburg, USA), sodium bicarbonate (Fisher Chemicals, Hampton, USA), and methylene blue (0.0002% w/v, Sigma-Aldrich). Water parameters were as following: temperature 28 ± 0.1 °C, pH 7.5 ± 0.1, conductivity 700 ± 50 μS/cm, ammonia and nitrites lower than 0.1 mg/L, and nitrates at 5 mg/L. Photoperiod was set to 14:10 h light–dark.
Waterborne exposure of zebrafish larvae to microalgae extracts
At 3 days post-fertilization (dpf), hatched larvae from AB and transgenic lines were transferred to 6-well plates at a density of 15 larvae/well, and exposed to ethanolic extracts, 0.1% ethanol (vehicle and negative control) or 10 pg/mL of calcitriol (1α,25-dihydroxy vitamin D3, Sigma-Aldrich; positive control) [33]. Treatment (10 mL/well) was renewed—70% of the total volume, i.e., 7 mL—daily until the end of the experiment. Mortality was monitored at the time of treatment renewal in order to establish the highest non-toxic concentration for each extract. At 6 dpf, larvae were killed with a lethal dose of MS-222 (0.6 mM, pH 7.0, Sigma-Aldrich), then stained for 20 min with 0.03% alizarin red S or 0.5% calcein prepared in Milli-Q water (pH 7.4; Millipore, Burlington, USA) and washed twice with Milli-Q water for 5 min. Larvae were imaged immediately upon staining. For gene expression analysis, 3-dpf larvae (AB line; 10 larvae per replicate and 3 replicates) were placed in a 100 × 15 mm Petri dish (Sarstedt) with 35 mL of treatment (prepared as described above). Larvae were exposed for 3 days to the highest non-toxic concentration of each extract, or the vehicle. At 6 dpf, fish from each Petri dish were pooled (10 fish per pool; 3 pools per condition) and processed for RNA extraction (see below).
Imaging and morphometric analysis
Euthanized larvae were placed in a lateral position on top of a 2.5% agarose gel plate and imaged using an MZ10F fluorescence stereomicroscope (Leica, Wetzlar, Germany) equipped with a DFC7000T color camera (Leica). A green fluorescence filter (λex = 546/10 nm) and a barrier filter (λem = 590 nm) were used to image AR-S stained wild-type larvae and Tg(sp7:mCherry) transgenic larvae, while a blue fluorescence filter (λex = 470/40 nm) and a barrier filter (λem = 515 nm) were used to image calcein stained wild-type larvae, and Tg(runx2:EGFP) and Tg(oc:EGFP) transgenic larvae. Images were acquired using the following parameters: exposure time of 300 ms (mCherry and AR-S) or 1 s (calcein and EGFP); gamma 1.00; resolution 1920 × 1440 pixels; binning 1 × 1. Images were analyzed using ImageJ (version 1.52p). For morphometric analysis, images were processed using ZFBONE macro-toolset for Fiji [34]. The area of the operculum (OpA), the area of the head (HA), the splanchnocranial area (SA), and the areas positive for reporter signals within the HA (sp7+, oc+) and within the SA (runx2+) were determined using an Intuos M drawing tablet (Wacom, Kazo, Japan). To correct for inter-specimen size variations OpA, sp7+ and oc+ were normalized using HA, and runx2+ was normalized using SA.
Preparation of feeds supplemented with ethanolic extracts
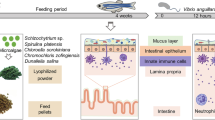
Ethanolic extracts SKLT and CTP4 were vacuum coated onto a zebrafish base diet (Sparos Lda, Olhão, Portugal) at 0.5% and 2.5% each. The five diets—CTRL (no extract), SKLT 0.5%, SKLT 2.5%, CTP4 0.5%, and CTP4 2.5% (Fig. 1B) were analyzed for phosphorus (P), calcium (Ca), potassium (K), and magnesium (Mg) contents. For this, diets were completely dried at 120 °C for 24 h in a VENTI-Line drying oven (VWR International, Radnor, USA), weighted and solubilized in nitric acid (Sigma-Aldrich). Mineral content was determined by microwave plasma atomic emission spectroscopy (4200 MP-AES, Agilent Technologies, Santa Clara, USA) using P, Ca, K, and Mg standards.
Dietary exposure to ethanolic extracts
At 3 dpf, zebrafish AB larvae were randomly distributed into 2.5 L tanks filled with water from the recirculating system filtered using 0.22 µm nylon membranes (Labbox) and maintained in static conditions at a density of 60 larvae/L. Environmental parameters were set as described above. Experimental procedures were conducted in triplicates for all conditions and the feeding regimen was adjusted with the growth of the fish (Fig. 1C). From 5 to 7 dpf, larvae were fed exclusively with live rotifers (Brachionus plicatilis). From 8 to 19 dpf, larvae were maintained in a regimen of co-feeding with rotifers and the experimental diets, gradually decreasing the number of rotifers and increasing the amount of dry food. From 20 to 55 dpf, fish were fed three times per day solely with the experimental diets. Food size was < 100 µm until 16 dpf and 100–200 µm until 55 dpf. At 20 dpf, 50 fish/tank were sampled to determine the total length (TL), while the remaining fish were transferred to 2.8 L glass tanks and maintained in recirculating water conditions until 55 dpf at a density of 36 larvae/L. At the end of the trial, juveniles were given a lethal anesthesia with MS-222 then randomly pooled for the assessment of dry weight (4 pools/replicate, 3 fish/pool), content of Ca and P (2 pools/replicate, 3 fish/pool), and gene expression by qPCR (2 pools/replicate, 3 fish/pool), as described below. The remaining fish (≥ 30 fish/replicate) were placed in a lateral position over a 2.5% agarose gel and photographed under a Leica MZ10F stereomicroscope equipped with a Leica DFC7000T color camera. Total length was assessed from bright-field images using ImageJ, then fish were fixed for 16 h in 4% paraformaldehyde prepared in phosphate-buffered saline (PBS, pH 7.4), then washed with PBS for 5 min and dehydrated in an increasing ethanol series. Subsequently, specimens were stained for 20 min with 0.05% AR-S in 1% KOH, then washed in 1% KOH for 48 h and preserved in glycerol. Incidence of skeletal anomalies and rate of skeleton mineralization were assessed in stained fish. To estimate the mineralization status of the skeleton, a color code was attributed to each skeletal element according to a mineralization index, where 0 is for elements with no mineralization, 1 for elements with a low level of mineralization, 2 for elements with intermediate levels of mineralization, and 3 for elements fully mineralized.
RNA extraction and qPCR analysis
Total RNA was extracted from pools of larvae at 6 dpf (n = 3, 10 larvae/pool) or juvenile fish at 55 dpf (n = 6, 3 fish/pool) using NZYol (NZYTech, Lisbon, Portugal) and quantified using a NanoDrop OneC spectrophotometer (ThermoFisher Scientific). RNA integrity was confirmed using an Experion Automated Electrophoresis system (Bio-Rad, Hercules, USA). Total RNA (1 μg) was reverse-transcribed for 1 h at 37 °C using M-MLV reverse transcriptase, oligo-d(T) primer and RNAseOUT (all from ThermoFisher Scientific). Quantitative real-time PCR (qPCR) reactions were performed using qPCR NZYSpeedy Mastermix (2x) ROX Plus (NZYTech), 10 μM of gene-specific primers (Supplementary Table I) and 1:10 dilution of reverse-transcribed RNA, in a CFX Connect Real-Time PCR detection system (Bio-Rad). PCR amplification was as follows: an initial denaturation step of 2 min at 95 °C and 40 cycles of amplification (10 s at 95 °C and 20 s at 65 °C). Efficiency of amplification was above 95% for all primer sets. Primer specificity was confirmed through sequence analysis and qPCR specificity was assessed by melting curve analysis at the end of each PCR run. Levels of gene expression were calculated using the ∆∆Ct method [38] and normalized using the average value of three housekeeping genes, ef1a, actb1 and rps18.
Statistical analysis
For all the experiments, normality was tested with a D’Agostino–Pearson omnibus normality test or with an Anderson–Darling test (p < 0.05). Homoscedasticity was tested through the Brown–Forsythe test (p < 0.05). If data distribution was normal and homogeneous in all the experimental groups, differences were tested with an unpaired t test (gene expression, p < 0.05) or a one-way ANOVA followed by Dunnett’s multiple comparison test (all other parameters, p < 0.05). If data distribution was not normal or not homogeneous, differences were tested with a Mann–Whitney test (gene expression, p < 0.05) or a non-parametric test followed by Dunn’s multiple comparison test (all other parameters, p < 0.05). Statistical analyses were performed using Prism version 8.00 (GraphPad Software Inc., La Jolla, USA).
Results
Microalgae extracts induce matrix mineralization in fish bone cell lines
As a first approach to evaluate the osteogenic potential of the ethanolic extracts prepared from Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4), mineralogenic cells VSa13 were exposed for 17 days to 3 concentrations of the two extracts. While SKLT increased ECM mineralization at the two highest concentrations (50 and 100 µg/mL; Fig. 2A), CTP4 showed a strong mineralogenic activity at all the concentrations tested (25, 100, and 300 µg/mL; Fig. 2B) and in a dose-dependent manner, indicating the presence of mineralogenic compounds in both extracts.
Mineralization of the extracellular matrix (ECM) of VSa13 cells exposed to ethanolic extracts prepared from A Skeletonema costatum (SKLT), and B Tetraselmis striata CTP4 (CTP4). Values are presented as mean ± standard deviation and as a percentage over the control group (Ethanol, n = 6). Picture panels above each graph display images of alizarin-red-stained cell cultures. Normality was tested through Anderson–Darling test (p < 0.05). Asterisks indicate values significantly different according to one-way ANOVA followed by post hoc Dunnett’s or Kruskal–Wallis test. Each experimental group was tested against the control group (Ethanol). p < 0.0001 (****). Min− No mineralogenic cocktail
Microalgae extracts promote bone formation by stimulating osteoblastic differentiation in vivo
The osteogenic potential of both extracts was assessed in zebrafish via waterborne exposure of 3-dpf larvae to different concentrations of SKLT and CTP4 followed by morphometric analysis of the opercular bone (Fig. 3A). Highest non-toxic concentrations—31.6 µg/mL for SKLT and 200 µg/mL for CTP4 (Supplementary Table II)—were established based on mortality records. Both extracts induced an increase of the mineralized area of the opercular bone in a dose-dependent manner (Fig. 3B and C), indicating the presence of osteogenic compounds. While CTP4 did not affect the head area (HA) at the concentrations tested, SKLT increased it at 31.6 µg/mL and 56 µg/mL, suggesting that other developmental processes (e.g., cranial development) might be affected (Supplementary Fig. 1). To investigate a possible role of osteoblasts in the pro-osteogenic capacity of microalgae extracts, cellular dynamics were monitored using transgenic reporter lines: Tg(runx2:EGFP) for osteo-chondroprogenitor cells, Tg(sp7:mCherry) for immature osteoblasts and, Tg(oc:EGFP) for mature osteoblasts. A significant increase in the signal associated with osterix/sp7 (sp7+ cells) and osteocalcin (oc+ cells), but not of runt related transcription factor 2 (runx2+ cells) was observed, suggesting that ethanolic extracts may increase operculum mineralized area through a specific action on committed osteoblast differentiation, but not on early differentiation of mesenchymal precursors (Fig. 4B, D, F). qPCR data confirmed that the expression of runx2a and runx2b was indeed unaffected in SKLT treated fish, while the expression of sp7 was increased. Accordingly, expression of col10a1a (collagen type X alpha 1a), whose expression is directly controlled by sp7, was upregulated in SKLT treated fish. In contrast, expression of sp7, oc1 and oc2 was not affected in CTP4-treated fish (Fig. 4G).
Mineralogenic effect on the operculum of 6-dpf zebrafish larvae exposed to Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4) extracts. A Composite image of a 6 dpf zebrafish larva, where operculum area (OpA) and head area (HA) are highlighted with a continuous line and a dashed line, respectively, and where AR-S stained mineralized structures detected by fluorescence microscopy appear in red. B, C Effects of SKLT (B) and CTP4 (C) on the operculum area. Values are presented as mean ± standard error and as a percentage over the negative control (Ethanol) (n > 15). The picture panels on the right side of each graph present images of opercular bones from negative and positive (calcitriol) control fish and fish exposed to the higher concentration of each extract. Normality was tested though Anderson–Darling test (p < 0.05). Asterisks indicate values significantly different according to one-way ANOVA followed by post hoc Dunnett’s or Kruskal–Wallis test. Each experimental group was tested against the negative control group (Ethanol). p < 0.0002 (***) and p < 0.0001 (****)
Expression of osteoblast differentiation markers in 6-dpf zebrafish larvae exposed to ethanolic extracts of Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4). Representative images of A runx2-positive osteochondroprogenitor cells, C sp7-positive immature osteoblasts, and E oc1-positive mature osteoblasts, and quantification of fluorescence signal area for runx2 (B), sp7 (D), and oc1 (E) positive cells. Normality was tested though Anderson–Darling test (p < 0.05). For fluorescence signals, differences were tested through one-way ANOVA or Kruskal–Wallis test. Asterisks indicate p < 0.0021 (**), p < 0.0002 (***) and p < 0.0001 (****). For gene expression, differences were tested through a non-parametric Kruskal–Wallis test, followed by a Dunn’s multiple comparison test (significant P values are reported above each treatment)
Dietary exposure to ethanolic extracts promotes bone formation and mineralization, and decrease the incidence of skeletal anomalies
To gain further insights into the osteogenic and osteoblastogenic effects of the extracts, zebrafish were fed diets supplemented with CTP4 and SKLT from 8 to 55 dpf. The five experimental diets produced were homogeneous in terms of proximal composition (Supplementary Table III) and content of P, Ca, K, and Mg (Supplementary Fig. 2). Fish fed SKLT 0.5% and CTP4 2.5% were larger than control fish at 20 dpf (Supplementary Fig. 3), but all fish had similar length and weight at 55 dpf. Our data suggest that none of the extracts significantly affected fish growth.
Although Ca and P contents did not significantly change in fish fed experimental diets (Fig. 5D), a general increase of the mineralization index attributed to each skeletal structure was observed in those fish. In fact, levels of mineralization increased in all the skeletal regions and structures considered and, in a dose-dependent manner for both extracts, with the fish fed SKLT 2.5% and CTP4 2.5% displaying the highest mineralization indexes (Fig. 5A–C). Accordingly, the expression of marker genes for osteoblast differentiation (sp7, oc1, oc2, col1a1a) was increased at 55 dpf in a dose-dependent manner and in fish fed the experimental diets (Fig. 5E). In opposition to the data collected after the short exposure, runx2b expression was downregulated in fish fed at both supplementation levels in SKLT diets. Interestingly, fish fed SKLT 2.5%, CTP4 0.5% and CTP4 2.5% also displayed an increased expression of acp5a, a marker gene for active osteoclasts and bone resorption (Fig. 5E), suggesting that extracts may also affect bone remodeling. The expression of marker genes for antioxidant function (cat and sod1) was also increased, suggesting a potentiation of the enzymatic removal of Reactive Oxygen Species (ROS) by the extracts (Fig. 6).
Mineralization status of zebrafish juveniles fed diets supplemented with the ethanolic extracts of Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4). A Heatmap displaying the group modal values for the mineralization index assigned to each skeletal structure. B Schematic representation of the modal mineralization status of the fish fed CTRL, SKLT 2.5% or CTP4 2.5% (from top to bottom). C Representative images of fish illustrated in C. D Content of phosphorus (P) and calcium (Ca), and Ca/P ratio in juveniles fed experimental diets. E Expression of marker genes for osteoblastic differentiation, ECM mineralization, and osteoclast function. For gene expression, differences were tested through Student’s t test. Asterisks indicate p < 0.0332 (*), p < 0.0021. (**), p < 0.0002 (***) and p < 0.0001 (****). J jaws, Op + Ba operculum and branchial arches, Vert vertebral bodies, NA + S neural arches and spines, HA + S haemal arches and spines, Uro caudal vertebrae bodies and urostyle
Effect of the ethanolic extracts of Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4) on the expression of antioxidant response markers in juvenile zebrafish (55 dpf). cat catalase, sod1 superoxide dismutase 1, soluble. Statistical differences between each group and the control (CTRL) were tested through Student’s t test (p < 0.05). Asterisks indicate values statistically different. p < 0.002 (**), p < 0.0002 (***), p < 0.0001 (****)
The occurrence of skeletal anomalies was assessed in AR-S stained fish and all groups displayed a similar distribution pattern, with opercular bones, abdominal vertebrae, caudal fin complex, and unpaired fins being the most affected structures (Supplementary Fig. 4). A more detailed analysis of the differences in incidence between the levels affecting the individual skeletal structures (Fig. 7A) showed that fish fed experimental diets, in particular SKLT 2.5% and CTP4 0.5%, had a lower incidence of anomalies compared to the control group (Fig. 7B). Interestingly, fish fed CTP4 2.5% had a lower incidence of caudal vertebrae anomalies and anomalies on all the five fins, but a higher incidence of anomalies affecting the skeletal elements associated with abdominal vertebrae (Fig. 7C).
Incidence of skeletal anomalies in zebrafish juveniles fed diets supplemented with the ethanolic extracts of Skeletonema costatum (SKLT) and Tetraselmis striata CTP4 (CTP4). A Scheme illustrating the skeletal elements considered in the analysis of skeletal anomalies. B Incidence of anomalies expressed as a percentage of increment/decrement of anomalies relative to the control group (non-supplemented diet). C Representative images of commonly found skeletal anomalies in AR-S stained fish for all experimental groups. Op + Ba operculum and branchial arches, Vert vertebral bodies, NA + S neural arches and spines, HA + S haemal arches and spines, Uro caudal vertebrae bodies and urostyle, LSK lordosis–scoliosis–kyphosis, DFI deformed fin lepidotrichia, A.Kyp abdominal kyphosis, C.Sco caudal scoliosis, HeA + S deformed haemal arches and spines
Discussion
Microalgae species belonging to the genera Skeletonema spp. and Tetraselmis spp. are commonly cultured worldwide as a food source due to favorable nutritional properties, e.g., high protein contents and diversified fatty acid profiles [39, 40]. Here, we have shown that ethanolic extracts from Skeletonema costatum and Tetraselmis striata (CTP4 strain) contain compounds with strong pro-osteoblastogenic and pro-mineralogenic activities using in vitro and in vivo fish models. Both extracts demonstrated pro-mineralogenic properties, an effect related to an increased rate of osteoblastic differentiation. This hypothesis is supported by in vivo data collected in zebrafish transgenic lines showing that both extracts selectively increased the populations of both immature (sp7+) and mature (oc+) osteoblasts, but not of osteoprogenitor cells (runx2+). This was further supported by expression data showing a reduced expression of runx2b in fish fed SKLT diets. Previous studies have shown that Runx2 regulates osteoblast differentiation in a dualistic manner. It induces the osteoblastic commitment of mesenchymal stem cells at early stages of differentiation but must be downregulated at the final stages of osteoblast maturation because of its capacity to maintain osteoblasts in an immature state [41,42,43,44]. We propose that the increase of sp7 and oc expression in larvae exposed to microalgae extracts and the decrease of runx2b expression observed in juveniles fed the same extracts is indicative of an accelerated osteoblast maturation that could explain the improved mineralogenic performances observed in exposed fish.
Furthermore, both the larvae exposed to the extracts for a short period (3 days) and the juveniles fed the extracts for a long period (50 days) showed a clear increase in the mineralization of skeletal elements (operculum for larvae and overall skeleton for juveniles). This suggests that the stimulation of osteoblastic differentiation by the microalgae extracts may be then translated into an increased mineralization status. We have also observed that both CTP4 and SKLT diets elevated the expression of genes involved in ECM formation (col10a1a, col1a1a) and resorption (acp5a), suggesting that compounds present in both extracts may have the ability to modulate bone remodeling. This process is highly regulated and governed by molecular programs that mitigate bone anabolic processes by the stimulation of osteoclastic differentiation, which is controlled by both osteoblasts and osteocytes through the RANK-RANKL-OPG signaling pathway [45]. The stimulation of resorptive processes is coherent with an overall increase of bone formation and osteoblastic maturation induced by the extracts. For example, human patients receiving treatment with PTH analogues display an increased osteoclastic differentiation, which is a secondary effect of the activation of anabolic mechanisms for a prolonged time [46].
Fish fed microalgae extracts were also characterized by a reduced incidence of skeletal anomalies in most skeletal elements, indicating an overall improvement of the skeleton health status. Still, an increase in skeletal anomalies associated to the accessory elements of abdominal vertebrae (ribs, neural arches and spines) was observed in fish fed CTP4 2.5%. It might not be a coincidence that this group was also the one presenting the stronger induction of mineralization. In this aspect, it has been shown in zebrafish that conditions that overstimulate bone mineralization, as it is the case for high levels of dietary phosphorus, may trigger an increased incidence of skeletal abnormalities [47].
In this work, phosphorus content was similar in all the experimental diets and cannot be responsible for the increased incidence of skeletal anomalies. Still, an excessive or excessively rapid ossification of the skeletal elements in fish fed CTP4 2.5% may be at the origin of an increased incidence of anomalies in these elements. Fish fed the CTP4 2.5% diet presented the highest degree of mineralization among all groups, and the accessory elements of the abdominal vertebrae showed the highest mineralization index reported. As such, a lower supplementation, for example at 0.5% as tested in this work, may represent a healthier option to provide a better balance between skeletal morphogenic processes and bone mineralization.
Interestingly, the expression of two marker genes of the antioxidant system—the catalase (cat) and superoxide dismutase (sod1)—was upregulated in fish exposed to both extracts (Fig. 6). It is important to mention that Skeletonema costatum and Tetraselmis striata CTP4 recently sparked the interest of the scientific community because of their high content in compounds with antioxidant activity, such as polyphenols [39, 48,49,50,51,52,53]. Ethanolic fractions of S. costatum and T. striata CTP4—rich in polyunsaturated fatty acids, alkanes and alkenes, long-chain alcohols, esters, ethers, and sterols—were also found to have in vitro radical scavenging activities [50, 52]. We hypothesize that the pro-osteogenic effects observed in fish exposed to microalgae extracts could be explained, at least in part, by the presence of antioxidant compounds in these extracts. Reactive oxygen species (ROS) are known to negatively affect bone mineral status and affect bone cells through several mechanisms, by inducing osteoclastic differentiation while suppressing osteoblastic differentiation and survival, being at the basis of various bone erosive pathologies including age-related osteoporosis [54, 55]. In this regard, the exposure of mammalian and fish models (in vivo and in vitro) to pro-oxidant agents have been shown to inhibit osteoblastic differentiation and activity and increased the incidence of skeletal anomalies, but the supplementation of antioxidants could counteract these negative outputs [56,57,58,59,60,61]. Recent studies reporting the osteoactive and antioxidative potential of hydroethanolic, methanolic, and dichloromethane extracts prepared from green (Cladophora rupestris and Codium fragile) and red (Ceramium secundatum, Ceramium pallidum, and Plocamium lyngbyanum) macroalgae, further support this hypothesis [5, 6, 62]. Similarly, polyphenol-rich extracts prepared from marine halophyte plants also triggered pro-osteogenic and pro-mineralogenic activities [7]. The increased expression of cat and sod1 in the present study may indicate a potentiation of fish antioxidant defenses upon exposure to microalgal extracts. In fact, many antioxidant agents exert their protective role against oxidative damage by upregulating the expression of first-line antioxidant enzymes [63,64,65]. However, this hypothesis should be further confirmed in future studies (i) by gathering data on protein levels and activity for Cat and Sod1, and on markers of cellular oxidative damage (e.g., DNA damage, lipid peroxidation, and loss in liposome membrane stability), but also (ii) by measuring the production of ROS in fish fed SKLT and CTP4 diets and challenged with pro-oxidant compounds.
Non-antioxidant compounds found in high amounts in microalgal biomass and known to stimulate bone morphogenesis and mineralization [66], may also be responsible for the pro-osteogenic effect observed here, e.g., polyunsaturated fatty acids [67, 68], phospholipids [69], liposoluble vitamins such as vitamin D, A and K [70,71,72], and minerals such as calcium, phosphorus, and magnesium [66, 72]. To identify the osteoactive compounds, extracts should be further fractionated and bioactive fractions should be characterized, e.g., through liquid chromatography coupled to mass spectrometry. Once identified and purified, these compounds should be further validated for applications in aquaculture nutrition as dietary supplements to ameliorate the skeletal health of reared fish species, or in the treatment of human bone erosive pathologies. Thus, future studies should aim at better characterizing the effect of these extracts in animal models that closely recapitulate the phenotypes of human pathologies. In this regard, zebrafish and medaka (Oryzias latipes) models of osteoporosis [24,25,26], osteomalacia [47], and Paget’s disease [73, 74] are available. Ovariectomized rats and mice are the most commonly used models of post-menopausal osteoporosis [75, 76], and rodent models of Paget’s disease are also available [77]. In addition, mammalian models resembling human diseases with secondary bone symptoms, such as vitamin D deficiency [78], hyperparathyroidism [79], and chronic kidney diseases [80] have been developed.
Overall, our data provide strong evidence for the presence of osteoactive compounds in ethanolic extracts of both microalgae, with the ability to stimulate bone mineralization by increasing osteoblast differentiation. The two species of marine microalgae evaluated, Skeletonema costatum and Tetraselmis striata CTP4, possess a high biotechnological potential as dietary supplement to ameliorate skeletal health in fish reared in aquaculture. In addition, these extracts might serve as a substrate for further identification of novel pharmaceuticals with applications in human medicine, although this will require a careful evaluation of the translatability of these results to mammalian systems that better model human bone disorders.
Availability of data and materials
All datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Boglione C, Gavaia P, Koumoundouros G, Gisbert E, Moren M, Fontagné S, Witten PE (2013) Skeletal anomalies in reared European fish larvae and juveniles. Part 1: normal and anomalous skeletogenic processes. Rev Aquac 5:S99–S120
Boglione C, Gisbert E, Gavaia P, Witten PE, Moren M, Fontagné S, Koumoundouros G (2013) Skeletal anomalies in reared European fish larvae and juveniles. Part 2: main typologies, occurrences and causative factors. Rev Aquac 5:S121–S167
Ryu B, Li YX, Kang KH, Kim SK, Kim DG (2015) Floridoside from Laurencia undulata promotes osteogenic differentiation in murine bone marrow mesenchymal cells. J Funct Foods 19:505–511
Carletti A, Cardoso C, Lobo-Arteaga J, Sales S, Juliao D, Ferreira I, Paula-Chainho I, Dionísio MA, Gaudêncio MJ, Afonso C, Lourenço H, Cancela ML, Bandarra NM, Gavaia PJ (2022) Antioxidant and anti-inflammatory extracts from sea cucumbers and tunicates induce a pro-osteogenic effect in zebrafish larvae. Front Nutr 9:833
Carson MA, Nelson J, Cancela ML, Laizé V, Gavaia PJ, Rae M, Heesch S, Verzin E, Maggs C, Gilmore BF, Clarke SA (2018) Red algal extracts from Plocamium lyngbyanum and Ceramium secundatum stimulate osteogenic activities in vitro and bone growth in zebrafish larvae. Sci Rep 8(1):1
Carson MA, Nelson J, Cancela ML, Laizé V, Gavaia PJ, Rae M, Heesch S, Verzin E, Maggs C, Gilmore BF, Clarke SA (2018) Screening for osteogenic activity in extracts from Irish marine organisms: The potential of Ceramium pallidum. PLoS ONE 13(11):0207303
Roberto VP, Surget G, Le Lann K, Mira S, Tarasco M, Guérard F, Poupart N, Laizé V, Stiger-Pouvreau V, Cancela ML (2021) Antioxidant, mineralogenic and osteogenic activities of Spartina alterniflora and Salicornia fragilis extracts rich in polyphenols. Front Nutr 8:555
Ringø E, Song SK (2016) Application of dietary supplements (synbiotics and probiotics in combination with plant products and β-glucans) in aquaculture. Aquac Nutr 22(1):4–24
Carrasco R, Fajardo C, Guarnizo P, Vallejo RA, Fernandez-Acero FJ (2018) Biotechnology applications of micro-algae in the context of EU “blue growth” initiatives. J Microbiol Genet 10:2574–7371
Hoang AT, Sirohi R, Pandey A, Nižetić S, Lam SS, Chen WH, Luque R, Thomas S, Arici M, Pham VV (2022) Biofuel production from microalgae: challenges and chances. Phytochem Rev 22:1089–1126
Pacheco D, Rocha AC, Pereira L, Verdelhos T (2020) Microalgae water bioremediation: trends and hot topics. Appl Sci 10(5):1886
Shah MR, Lutzu GA, Alam A, Sarker P, Kabir Chowdhury MA, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Saadaoui I, Rasheed R, Aguilar A, Cherif M, Al Jabri H, Sayadi S, Manning SR (2021) Microalgal-based feed: promising alternative feedstocks for livestock and poultry production. J Anim Sci Biotechnol 12(1):76
Torres-Tiji Y, Fields FJ, Mayfield SP (2020) Microalgae as a future food source. Biotechnol Adv 41:107536
Sathasivam R, Radhakrishnan R, Hashem A, Abdallah EF (2019) Microalgae metabolites: a rich source for food and medicine. Saudi J Biol Sci 26(4):709
Kratzer R, Murkovic M (2021) Food ingredients and nutraceuticals from microalgae: main product classes and bio-technological production. Foods 10(7):1626
Jha D, Jain V, Sharma B, Kant A, Garlapati VK (2017) Microalgae-based pharmaceuticals and nutraceuticals: an emerging field with immense market potential. ChemBioEng Rev 4(4):257
Saide A, Martínez KA, Ianora A, Lauritano C (2021) Unlocking the health potential of microalgae as sustainable sources of bioactive compounds. Int J Mol Sci 22:4383
Sproles AE, Fields FJ, Smalley TN, Le CH, Badary A, Mayfield SP (2021) Recent advancements in the genetic engineering of microalgae. Algal Res 53:102158
Yan N, Fan C, Chen Y, Hu Z (2016) The potential for microalgae as bioreactors to produce pharmaceuticals. Int J Mol Sci 17(6):962
Shi Q, Chen C, Zhang W, Wu P, Sun M, Wu H, Wu H, Fu P, Fan J (2021) Transgenic eukaryotic microalgae as green factories: providing new ideas for the production of biologically active substances. J Appl Phycol 33:705
Laizé V, Gavaia PJ, Cancela ML (2014) Fish: a suitable system to model human bone disorders and discover drugs with osteogenic or osteotoxic activities. Drug Discov Today: Dis Model 13:29
Rosa JT, Tarasco M, Gavaia PJ, Cancela ML, Laizé V (2022) Screening of mineralogenic and osteogenic compounds in zebrafish—tools to improve assay throughput and data accuracy. Pharmaceuticals 15:983
Bergen DJ, Kague E, Hammond CL (2019) Zebrafish as an emerging model for osteoporosis: a primary testing platform for screening new osteo-active compounds. Front Endocrinol 10:6
Rosa JT, Laizé V, Gavaia PJ, Cancela ML (2021) Fish models of induced osteoporosis. Front Cell Dev Biol 9:672424
Lleras-Forero L, Winkler C, Schulte-Merker S (2020) Zebrafish and medaka as models for biomedical research of bone diseases. Dev Biol 457(2):191
Spoorendonk KM, Hammond CL, Huitema LF, Vanoevelen J, Schulte-Merker S (2010) Zebrafish as a unique model system in bone research: the power of genetics and in vivo imaging. J Appl Ichthyol 26(2):219
Ulloa PE, Medrano JF, Feijoo CG (2014) Zebrafish as animal model for aquaculture nutrition research. Front Genet 5:313
Jørgensen LVG (2020) Zebrafish as a model for fish diseases in aquaculture. Pathogens 9(8):609
Pombinho AR, Laizé V, Molha DM, Marques SM, Cancela ML (2004) Development of two bone-derived cell lines from the marine teleost Sparus aurata; evidence for extracellular matrix mineralization and cell-type-specific expression of matrix Gla protein and osteocalcin. Cell Tissue Res 315(3):393
Marques CL, Rafael MS, Cancela ML, Laizé V (2007) Establishment of primary cell cultures from fish calcified tissues. Cytotechnology 55:9
Stanford CM, Jacobson PA, Eanes ED, Lembke LA, Midura RJ (1995) Rapidly forming apatitic mineral in an osteoblastic cell line. J Biol Chem 270(16):9420
Tarasco M, Laizé V, Cardeira J, Cancela ML, Gavaia PJ (2017) The zebrafish operculum: a powerful system to assess osteogenic bioactivities of molecules with pharmacological and toxicological relevance. Comp Biochem Physiol Part C: Toxicol Pharmacol 197:45
Tarasco M, Cordelières FP, Cancela ML, Laizé V (2020) ZFBONE: An ImageJ toolset for semi-automatic analysis of zebrafish bone structures. Bone 138:115480
Cardeira J, Gavaia PJ, Fernández I, Cengiz I-F, Moreira-Silva J, Oliveira JM, Reis R, Cancela ML, Laizé V (2016) Quantitative assessment of the regenerative and mineralogenic performances of the zebrafish caudal fin. Sci Rep 6:39191
Knopf F, Hammond C, Chekuru A, Kurth T, Hans S, Weber CW, Mahatma G, Fisher S, Brand M, Schulte-Merker S, Weidinger G (2011) Bone regenerates via de-differentiation of osteoblasts in the zebrafish fin. Dev Cell 20(5):713
Singh SP, Holdway SP, Poss KD (2012) Regeneration of amputated zebrafish fin rays from de novo osteoblasts. Dev Cell 22(4):879
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29(9):45
De Jesus Raposo MF, De Morais RM, De Morais AM (2013) Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar Drugs 11(1):233
Becker EW (2013) Microalgae for aquaculture: nutritional aspects. Handbook of microalgal culture applied phycology and biotechnology. Wiley, pp 671–691
Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G (2004) A twist code determines the onset of osteoblast differentiation. Dev Cell 6(3):423
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99(5):1233
Komori T (2010) Regulation of osteoblast and odontoblast differentiation by runx2. J Oral Biosci 52(1):22
Bruderer M, Richards RG, Alini M, Stoddart MJ (2014) Role and regulation of RUNX2 in osteogenesis. Eur Cell Mater 28:269
Kenkre JS, Bassett JHD (2018) The bone remodelling cycle. Ann Clin Biochem 55:308
Tabacco G, Bilezikian JP (2019) Osteoanabolic and dual action drugs. Br J Clin Pharmacol 85:1084
Cotti S, Huysseune A, Koppe W, Rücklin M, Marone F, Wölfel EM, Fiedler IAK, Busse B, Forlino A, Witten PE (2020) More bone with less minerals? The effects of dietary phosphorus on the post-cranial skeleton in zebrafish. Int J Mol Sci 21(15):5429
Cardoso C, Pereira H, Franca J, Matos J, Monteiro I, Pousão-Ferreira P, Gomes A, Barreira L, Varela J, Neng N, Nogueira J, Afonso C, Bandarra NM (2020) Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac Int 28:711
Bhattacharjya R, Marella TK, Tiwari A, Saxena A, Singh PK, Mishra B (2020) Bioprospecting of marine diatoms Thalassiosira, Skeletonema and Chaetoceros for lipids and other value-added products. Bioresour Technol 318:124073
Guarda I, Fonseca I, Pereira H, Martins LL, Gomes R, Matos J, Gomes-Bispo A, Bandarra NM, Afonso C, Cardoso C (2021) Key constituents and antioxidant activity of novel functional foods developed with Skeletonema sp. biomass. J Aquat Food Prod Technol 30(9):1189
Guillerme JB, Couteau C, Coiffard L (2017) Applications for marine resources in cosmetics. Cosmetics 4(3):35
Sedjati S, Pringgenies D, Fajri M (2020) Determination of the pigment content and antioxidant activity of the marine microalga Tetraselmis suecica. Jordan J Biol Sci 13(1):55
Silva M, Kamberovic F, Uota ST, Kovan IM, Viegas CS, Simes DC, Barreira L (2022) Microalgae as potential sources of bioactive compounds for functional foods and pharmaceuticals. Appl Sci 12(12):5877
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33:359
Banfi G, Iorio EL, Corsi MM (2008) Oxidative stress, free radicals and bone remodeling. Clin Chem Lab Med 46(11):1550
Izquierdo MS, Scolamacchia M, Betancor M, Roo J, Caballero MJ, Terova G, Witten PE (2013) Effects of dietary DHA and α-tocopherol on bone development, early mineralisation and oxidative stress in Sparus aurata (Linnaeus, 1758) larvae. Br J Nutr 109(10):1796
Birnie-Gauvin K, Costantini D, Cooke SJ, Willmore WG (2017) A comparative and evolutionary approach to oxidative stress in fish: a review. Fish Fish 18(5):928
Manolagas SC (2010) From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis. Endocr Rev 31(3):266
Poudel S, Martins G, Cancela ML, Gavaia PJ (2022) Regular supplementation with antioxidants rescues doxorubicin-induced bone deformities and mineralization delay in zebrafish. Nutrients 14:4959
Poudel S, Izquierdo M, Cancela ML, Gavaia PJ (2022) Reversal of doxorubicin-induced bone loss and mineralization by supplementation of resveratrol and mitoTEMPO in the early development of Sparus Aurata. Nutrients 14:1154
Poudel S, Martins G, Cancela ML, Gavaia PJ (2022) Resveratrol-mediated reversal of doxorubicin-induced osteoclast differentiation. Int J Mol Sci 23(23):15160
Surget G, Roberto VP, Le Lann K, Mira S, Guérard F, Laizé V, Poupart N, Cancela ML, Stiger-Pouvreau V (2017) Marine green macroalgae: a source of natural compounds with mineralogenic and antioxidant activities. J Appl Phycol 29:575
Puiggròs F, Llópiz N, Ardévol A, Bladé C, Arola L, Salvadó MJ (2005) Grape seed procyanidins prevent oxidative injury by modulating the expression of antioxidant enzyme systems. J Agric Food Chem 53:6080
Leite MF, De Lima A, Massuyama MM, Otton R (2010) In vivo astaxanthin treatment partially prevents antioxidant alterations in dental pulp from alloxan-induced diabetic rats. Int Endod J 43(11):959
Arjunan P, Lin X, Tang Z, Du Y, Kumar A, Liu L, Yin X, Huang L, Chen W, Chen Q, Ye Z, Wang S, Kuang H, Zhou L, Xu K, Chen X, Zeng H, Lu W, Cao Y, Liu Y, Zhao C, Li X (2018) VEGF-B is a potent antioxidant. Proc Natl Acad Sci 115(41):10351
Hamre K, Yufera M, Rønnestad I, Boglione C, Conceição LE, Izquierdo M (2013) Fish larval nutrition and feed formulation: knowledge gaps and bottlenecks for advances in larval rearing. Rev Aquac 5:S26–S58
Roo J, Hernández-Cruz CM, Mesa-Rodriguez A, Fernández-Palacios H, Izquierdo MS (2019) Effect of increasing n-3 HUFA content in enriched Artemia on growth, survival and skeleton anomalies occurrence of greater amberjack Seriola dumerili larvae. Aquaculture 500:651–659
Sargent JR, Tocher DR, Bell JG (2002) The lipids. In: Halver JE, Hardy RW (eds) Fish nutrition, 3rd edn. Elsevier, pp 181–257
Kanazawa A, Teshima S, Inamori S, Iwashita T, Nagao A (1981) Effects of phospholipids on growth, survival rate and incidence of malformation in the larval ayu. Mem Fac Fish Kagoshima Univ 30:301–309
Lock EJ, Waagbø R, Wendelaar-Bonga S, Flik G (2010) The significance of vitamin D for fish: a review. Aquac Nutr 16(1):100–116
Mazurais D, Glynatsi N, Darias MJ, Christodoulopoulou S, Cahu CL, Zambonino-Infante JL, Koumoundouros G (2009) Optimal levels of dietary vitamin A for reduced deformity incidence during development of European sea bass larvae (Dicentrarchus labrax) depend on malformation type. Aquaculture 294(3–4):262–270
Lall SP, Lewis-McCrea LM (2007) Role of nutrients in skeletal metabolism and pathology in fish—an overview. Aquaculture 267(1–4):3–19
Silva IAL, Conceição N, Michou L, Cancela ML (2014) Can zebrafish be a valid model to study Paget’s disease of bone? J Appl Ichthyol 30(4):678
Huybrechts Y, De Ridder R, De Samber B, Boudin E, Tonelli F, Knapen D, Schepers D, De Beenhouwer J, Sijbers J, Forlino A, Mortier G, Coucke P, Witten PE, Kwon R, Willaert A, Hendrickx G, Van Hul W (2022) The sqstm1tmΔUBA zebrafish model, a proof-of-concept in vivo model for Paget’s disease of bone? Bone Rep 16:101483
Sophocleous A, Idris AI (2014) Rodent models of osteoporosis. BoneKEy Rep 3:614
Yousefzadeh N, Kashfi K, Jeddi S, Ghasemi A (2020) Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J 19:89
Alonso N, Wani S, Rose L, Van’t Hof RJ, Ralston SH, Albagha OM (2021) Insertion mutation in Tnfrsf11a causes a Paget’s disease-like phenotype in heterozygous mice and osteopetrosis in homozygous mice. J Bone Miner Res 36(7):1376
Stavenuiter AW, Arcidiacono MV, Ferrantelli E, Keuning ED, Vila Cuenca M, Ter Wee PM, Beelen RHJ, Vervloet MG, Dusso AS (2015) A novel rat model of vitamin D deficiency: safe and rapid induction of vitamin D and calcitriol deficiency without hyperparathyroidism. BioMed Res Int. https://doi.org/10.1155/2015/604275
Jaeger P, Jones W, Kashgarian M, Baron R, Clemens TL, Segre GV, Hayslett JP (1987) Animal model of primary hyperparathyroidism. Am J Physiol-Endocrinol Metab 252(6):790
Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH II (2009) A rat model of chronic kidney disease-mineral bone disorder. Kidney Int 75(2):176
Acknowledgements
All funding organizations are listed below the “Funding” headline. All the authors acknowledge João Navalho, Necton SA (Olhão, Portugal), for making available microalgal biomass, and Jorge Dias, Sparos Lda (Olhão, Portugal), for producing the extracts-supplemented diets for the dietary trials.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was financed by the European Maritime and Fisheries Fund (EMFF/FEAMP) through the National Operational Programme MAR2020 (grant 16-02-01-FMP-0057/OSTEOMAR), by the European Regional Development Fund (ERDF/FEDER) through the Transnational Cooperation Programme Atlantic Area (grant EAPA/151/2016/BLUEHUMAN), by the Marie Skłodowska-Curie innovative training network BIOMEDAQU (grant H2020-MSCA-ITN/766347), by National funds through the Portuguese Foundation for Science and Technology (grants UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020, and doctoral fellowships 2021.05406.BD and SFRH/BD/140143/2018) and by the operational programmes CRESC Algarve 2020 and COMPETE 2020 through project EMBRC.PT ALG-01-0145-FEDER-022121.
Author information
Authors and Affiliations
Contributions
AC, JTR, PJG, and VL: conceptualization and investigation. AC, JTR, KP, IB, TS, PJG, and VL: methodology. JV, LB, TS, HP, MLC, PJG, and VL: resources. AC, JTR, KP, and IB: data curation. AC, JTR, PJG, and VL: writing. AC, JTR, KP, IB, LB, TS, HP, MLC, PJG, and VL: review and editing. LB, MLC, PJG, and VL: supervision. AC, MLC, PJG, and VL: funding acquisition. All the authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Ethical approval
Procedures involving animals were performed following the EU and Portuguese legislation for animal experimentation and welfare (Directives 86/609/CEE and 2010/63/EU; Portaria 1005/92, 466/95 and 1131/97; Decreto-Lei 113/2013). Animal handling and experimentation were performed by qualified operators accredited by the Portuguese Direção-Geral de Alimentação e Veterinária under the authorization no. 012769/2021. All efforts were made to minimize pain, distress, and discomfort. Experiments were terminated (fish were returned to normal conditions or euthanized) whenever adverse effects were observed.
Consent for publication
No human research participants were involved in the present study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carletti, A., Rosa, J.T., Pes, K. et al. The osteogenic and mineralogenic potential of the microalgae Skeletonema costatum and Tetraselmis striata CTP4 in fish models. Cell. Mol. Life Sci. 80, 310 (2023). https://doi.org/10.1007/s00018-023-04953-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04953-y