Abstract
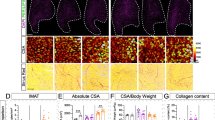
Both adipose tissue and skeletal muscle are highly dynamic tissues and interact at the metabolic and hormonal levels in response to internal and external stress, and they coordinate in maintaining whole-body metabolic homeostasis. In our previous study, we revealed that adipocyte-specific Rnf20 knockout mice (ASKO mice) exhibited lower fat mass but higher lean mass, providing a good model for investigating the adipose-muscle crosstalk and exploring the effect of the adipocyte Rnf20 gene on the physiology and metabolism of skeletal muscle. Here, we confirmed that ASKO mice exhibited the significantly increased body weight and gastrocnemius muscle weight. Fiber-type switching in the soleus muscle of ASKO mice was observed, as evidenced by the increased number of fast-twitch fibers and decreased number of slow-twitch fibers. Serum metabolites with significant alteration in abundance were identified by metabolomic analysis and the elevated lysophosphatidylcholine 16:0 [LysoPC (16:0)] was observed in ASKO mice. In addition, lipidome analysis of gonadal white adipose tissue revealed a significant increase in LysoPCs and LysoPC (16:0) in ASKO mice. Furthermore, knockdown of Rnf20 gene in 3T3-L1 cells significantly increased the secretion of LysoPC, suggesting that LysoPC might be a critical metabolite in the adipose-muscle crosstalk of ASKO mice. Furthermore, in vitro study demonstrated that LysoPC (16:0) could induce the expression of fast-twitch muscle fibers related genes in differentiated C2C12 cells, indicating its potential role in adipose-muscle crosstalk. Taken together, these findings not only expand our understanding of the biological functions of Rnf20 gene in systemic lipid metabolism, but also provide insight into adipose tissue dysfunction-induced physiological alterations in skeletal muscle.







Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
Abbreviations
- RNF20:
-
Ring finger 20
- WT mice:
-
Wild-type mice
- ASKO mice:
-
Adipocyte-specific Rnf20 knockout mice
- ERK1/2:
-
Extracellular regulated protein kinase 1/2
- GLUT4:
-
Glucose transporter type 4
- IRS-1:
-
Insulin receptor substrate-1
- SREBP1c:
-
Sterol regulatory element binding protein 1c
- NCoR1:
-
Nuclear receptor corepressor 1
- FFA:
-
Free fatty acid
- LPA:
-
Lysophosphatidic acid
- gWAT:
-
Gonadal white adipose tissue
- PBST:
-
Phosphate buffered solution
- BSA:
-
Bovine serum albumin
- CSA:
-
Cross-sectional area
- TAG:
-
Triacylglycerol
- DAG:
-
Diacylglycerol
- CE:
-
Cholesteryl esters
- PC:
-
Phosphatidylcholine
- PE:
-
Phosphatidylethanolamine
- PS:
-
Phosphatidylserine
- LysoPC:
-
Lysophosphatidylcholine
- LysoPE:
-
Lysophosphatidylethanolamine
- LysoPS:
-
Lysophosphatidylserine
- SM:
-
Sphingomyelin
- qRT-PCR:
-
Quantitative reverse transcription PCR
- FBS:
-
Fetal bovine serum
- PCA:
-
Principal component analysis
- FC:
-
Fold change
- PUFA:
-
Polyunsaturated fatty acids
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- SCD-1:
-
Stearoyl-CoA desaturase-1
- PLA2:
-
Phospholipase A2
- Chk:
-
Choline kinase
- Ctα:
-
Phosphocholine cytidylyltransferase α
- Chpt:
-
Choline phosphotransferase
References
Relaix F, Bencze M, Borok MJ, Der Vartanian A, Gattazzo F, Mademtzoglou D, Perez-Diaz S, Prola A, Reyes-Fernandez PC, Rotini A, Taglietti T (2021) Perspectives on skeletal muscle stem cells. Nat Commun 121:692. https://doi.org/10.1038/s41467-020-20760-6
Blaauw B, Schiaffino S, Reggiani C (2013) Mechanisms modulating skeletal muscle phenotype. Compr Physiol 34:1645–1687. https://doi.org/10.1002/cphy.c130009
Zierath JR, Hawley JA (2004) Skeletal muscle fiber type: influence on contractile and metabolic properties. PLoS Biol 210:e348. https://doi.org/10.1371/journal.pbio.0020348
Schiaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 914:1447–1531. https://doi.org/10.1152/physrev.00031.2010
Wang T, Xu YQ, Yuan YX, Xu PW, Zhang C, Li F, Wang LN, Yin C, Zhang L, Cai XC, Zhu CJ, Xu JR, Liang BQ, Schaul S, Xie PP, Yue D, Liao ZR, Yu LL, Luo L, Zhou G, Yang JP, He ZH, Du M, Zhou YP, Deng BC, Wang SB, Gao P, Zhu XT, Xi QY, Zhang YL, Shu G, Jiang QY (2019) Succinate induces skeletal muscle fiber remodeling via Suncr1 signaling. EMBO Rep 209:e47892. https://doi.org/10.15252/embr.201947892
Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 88:457–465. https://doi.org/10.1038/nrendo.2012.49
Ukropec J, Ukropcova B, Kurdiova T, Gasperikova D, Klimes I (2008) Adipose tissue and skeletal muscle plasticity modulates metabolic health. Arch Physiol Biochem 1145:357–368. https://doi.org/10.1080/13813450802535812
Fan HQ, Gu N, Liu F, Fei L, Pan XQ, Guo M, Chen RH, Guo XR (2007) Prolonged exposure to resistin inhibits glucose uptake in rat skeletal muscles. Acta Pharmacol Sin 283:410–416. https://doi.org/10.1111/j.1745-7254.2007.00523.x
Muoio DM, Dohm GL, Fiedorek FT Jr, Tapscott EB, Coleman RA (1997) Leptin directly alters lipid partitioning in skeletal muscle. Diabetes 468:1360–1363. https://doi.org/10.2337/diab.46.8.1360
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T (2002) Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating amp-activated protein kinase. Nat Med 811:1288–1295. https://doi.org/10.1038/nm788
Fong Y, Moldawer LL, Marano M, Wei H, Barber A, Manogue K, Tracey KJ, Kuo G, Fischman DA, Cerami A et al (1989) Cachectin/Tnf or Il-1 alpha induces cachexia with redistribution of body proteins. Am J Physiol 2563(Pt 2):R659–R665. https://doi.org/10.1152/ajpregu.1989.256.3.R659
Funcke JB, Scherer PE (2019) Beyond adiponectin and leptin: adipose tissue-derived mediators of inter-organ communication. J Lipid Res 6010:1648–1684. https://doi.org/10.1194/jlr.R094060
Zhou Q, Du J, Hu Z, Walsh K, Wang XH (2007) Evidence for adipose-muscle cross talk: opposing regulation of muscle proteolysis by adiponectin and fatty acids. Endocrinology 14812:5696–5705. https://doi.org/10.1210/en.2007-0183
Rancoule C, Attane C, Gres S, Fournel A, Dusaulcy R, Bertrand C, Vinel C, Treguer K, Prentki M, Valet P, Saulnier-Blache JS (2013) Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia 566:1394–1402. https://doi.org/10.1007/s00125-013-2891-3
Samad F, Hester KD, Yang G, Hannun YA, Bielawski J (2006) Altered adipose and plasma sphingolipid metabolism in obesity: a potential mechanism for cardiovascular and metabolic risk. Diabetes 559:2579–2587. https://doi.org/10.2337/db06-0330
Vantyghem MC, Pigny P, Maurage CA, Rouaix-Emery N, Stojkovic T, Cuisset JM, Millaire A, Lascols O, Vermersch P, Wemeau JL, Capeau J, Vigouroux C (2004) Patients with familial partial lipodystrophy of the dunnigan type due to a Lmna R482w mutation show muscular and cardiac abnormalities. J Clin Endocrinol Metab 8911:5337–5346. https://doi.org/10.1210/jc.2003-031658
Sellers RS, Mahmood SR, Perumal GS, Macaluso FP, Kurland IJ (2019) Phenotypic modulation of skeletal muscle fibers in Lpin1-deficient lipodystrophic ( Fld) mice. Vet Pathol 562:322–331. https://doi.org/10.1177/0300985818809126
Xu W, Zhou H, Xuan H, Saha P, Wang G, Chen W (2019) Novel metabolic disorders in skeletal muscle of lipodystrophic Bscl2/seipin deficient mice. Mol Cell Endocrinol 482:1–10. https://doi.org/10.1016/j.mce.2018.12.001
Fuchs G, Oren M (2014) Writing and reading H2b monoubiquitylation. Biochim Biophys Acta 18398:694–701. https://doi.org/10.1016/j.bbagrm.2014.01.002
Ren P, Sheng Z, Wang Y, Yi X, Zhou Q, Zhou J, Xiang S, Hu X, Zhang J (2014) Rnf20 promotes the polyubiquitination and proteasome-dependent degradation of Ap-2alpha protein. Acta Biochim Biophys Sin (Shanghai) 462:136–140. https://doi.org/10.1093/abbs/gmt136
Lee JH, Jeon YG, Lee KH, Lee HW, Park J, Jang H, Kang M, Lee HS, Cho HJ, Nam DH, Kwak C, Kim JB (2017) Rnf20 suppresses tumorigenesis by inhibiting the Srebp1c-Pttg1 axis in kidney cancer. Mol Cell Biol. https://doi.org/10.1128/MCB.00265-17
Jeon YG, Lee JH, Ji Y, Sohn JH, Lee D, Kim DW, Yoon SG, Shin KC, Park J, Seong JK, Cho JY, Choe SS, Kim JB (2020) Rnf20 functions as a transcriptional coactivator for ppargamma by promoting Ncor1 degradation in adipocytes. Diabetes 691:20–34. https://doi.org/10.2337/db19-0508
Zhao Y, Pan J, Cao C, Liang X, Yang S, Liu L, Tao C, Zhao J, Wang Y (2021) Rnf20 affects porcine adipocyte differentiation via regulation of mitotic clonal expansion. Cell Prolif 5412:e13131. https://doi.org/10.1111/cpr.13131
Liang X, Tao C, Pan J, Zhang L, Liu L, Zhao Y, Fan Y, Cao C, Liu J, Zhang J, Lam SM, Shui G, Jin W, Li W, Zhao J, Li K, Wang Y (2021) Rnf20 deficiency in adipocyte impairs adipose tissue development and thermogenesis. Protein Cell 126:475–492. https://doi.org/10.1007/s13238-020-00770-2
Pan J, Tao C, Cao C, Zheng Q, Lam SM, Shui G, Liu X, Li K, Zhao J, Wang Y (2019) Adipose lipidomics and Rna-Seq analysis revealed the enhanced mitochondrial function in Ucp1 knock-in pigs. Biochim Biophys Acta Mol Cell Biol Lipids 186410:1375–1383. https://doi.org/10.1016/j.bbalip.2019.06.017
Pereyra AS, Lin CT, Sanchez DM, Laskin J, Spangenburg EE, Neufer PD, Fisher-Wellman K, Ellis JM (2022) Skeletal muscle undergoes fiber type metabolic switch without myosin heavy chain switch in response to defective fatty acid oxidation. Mol Metab. 59:101456. https://doi.org/10.1016/j.molmet.2022.101456
Wang L, Zhou M (2023) Structure of a eukaryotic cholinephosphotransferase-1 reveals mechanisms of substrate recognition and catalysis. Nat Commun 141:2753. https://doi.org/10.1038/s41467-023-38003-9
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD (2001) Akt/Mtor pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 311:1014–1019. https://doi.org/10.1038/ncb1101-1014
Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ (2001) Mediation of Igf-1-induced skeletal myotube hypertrophy by Pi(3)K/Akt/Mtor and Pi(3)K/Akt/Gsk3 pathways. Nat Cell Biol 311:1009–1013. https://doi.org/10.1038/ncb1101-1009
Ryu YC, Choi YM, Lee SH, Shin HG, Choe JH, Kim JM, Hong KC, Kim BC (2008) Comparing the histochemical characteristics and meat quality traits of different pig breeds. Meat Sci 802:363–369. https://doi.org/10.1016/j.meatsci.2007.12.020
Shi H, Scheffler JM, Pleitner JM, Zeng C, Park S, Hannon KM, Grant AL, Gerrard DE (2008) Modulation of skeletal muscle fiber type by mitogen-activated protein kinase signaling. FASEB J 228:2990–3000. https://doi.org/10.1096/fj.07-097600
Koh HJ, Brandauer J, Goodyear LJ (2008) Lkb1 and Ampk and the regulation of skeletal muscle metabolism. Curr Opin Clin Nutr Metab Care 113:227–232. https://doi.org/10.1097/MCO.0b013e3282fb7b76
Frayn KN (2002) Adipose tissue as a buffer for daily lipid flux. Diabetologia 459:1201–1210. https://doi.org/10.1007/s00125-002-0873-y
D’Souza K, Nzirorera C, Cowie AM, Varghese GP, Trivedi P, Eichmann TO, Biswas D, Touaibia M, Morris AJ, Aidinis V, Kane DA, Pulinilkunnil T, Kienesberger PC (2018) Autotaxin-Lpa signaling contributes to obesity-induced insulin resistance in muscle and impairs mitochondrial metabolism. J Lipid Res 5910:1805–1817. https://doi.org/10.1194/jlr.M082008
Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS (2008) Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 1346:933–944. https://doi.org/10.1016/j.cell.2008.07.048
Lynes MD, Leiria LO, Lundh M, Bartelt A, Shamsi F, Huang TL, Takahashi H, Hirshman MF, Schlein C, Lee A, Baer LA, May FJ, Gao F, Narain NR, Chen EY, Kiebish MA, Cypess AM, Bluher M, Goodyear LJ, Hotamisligil GS, Stanford KI, Tseng YH (2017) The cold-induced lipokine 12,13-dihome promotes fatty acid transport into brown adipose tissue. Nat Med 235:631–637. https://doi.org/10.1038/nm.4297
Lin H, Long JZ, Roche AM, Svensson KJ, Dou FY, Chang MR, Strutzenberg T, Ruiz C, Cameron MD, Novick SJ, Berdan CA, Louie SM, Nomura DK, Spiegelman BM, Griffin PR, Kamenecka TM (2018) Discovery of hydrolysis-resistant isoindoline N-acyl amino acid analogues that stimulate mitochondrial respiration. J Med Chem 617:3224–3230. https://doi.org/10.1021/acs.jmedchem.8b00029
Schafer N, Yu Z, Wagener A, Millrose MK, Reissmann M, Bortfeldt R, Dieterich C, Adamski J, Wang-Sattler R, Illig T, Brockmann GA (2014) Changes in metabolite profiles caused by genetically determined obesity in mice. Metabolomics 103:461–472. https://doi.org/10.1007/s11306-013-0590-1
Ferrara PJ, Verkerke ARP, Maschek JA, Shahtout JL, Siripoksup P, Eshima H, Johnson JM, Petrocelli JJ, Mahmassani ZS, Green TD, McClung JM, Cox JE, Drummond MJ, Funai K (2021) Low lysophosphatidylcholine induces skeletal muscle myopathy that is aggravated by high-fat diet feeding. FASEB J 3510:e21867. https://doi.org/10.1096/fj.202101104R
Boon MR, Bakker LEH, Prehn C, Adamski J, Vosselman MJ, Jazet IM, Arias-Bouda LMP, van Lichtenbelt WDM, van Dijk KW, Rensen PCN, Mook-Kanamori DO (2017) Lysopc-Acyl C16:0 is associated with brown adipose tissue activity in men. Metabolomics 135:48. https://doi.org/10.1007/s11306-017-1185-z
Sekas G, Patton GM, Lincoln EC, Robins SJ (1985) Origin of plasma lysophosphatidylcholine: evidence for direct hepatic secretion in the rat. J Lab Clin Med 1052:190–194
Mehedint MG, Zeisel SH (2013) Choline’s role in maintaining liver function: new evidence for epigenetic mechanisms. Curr Opin Clin Nutr Metab Care 163:339–345. https://doi.org/10.1097/MCO.0b013e3283600d46
Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y, Huggins LA, Eriksson JW, Buckett LK, Turnbull AV, Ginsberg HN, Blaner WS, Huang LS, Goldberg IJ (2011) Dgat1 deficiency decreases Ppar expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J Lipid Res 524:732–744. https://doi.org/10.1194/jlr.M011395
Schlegel C, Lapierre LA, Weis VG, Williams JA, Kaji I, Pinzon-Guzman C, Prasad N, Boone B, Jones A, Correa H, Levy SE, Han X, Wang M, Thomsen K, Acra S, Goldenring JR (2018) Reversible deficits in apical transporter trafficking associated with deficiency in diacylglycerol acyltransferase. Traffic 1911:879–892. https://doi.org/10.1111/tra.12608
Hall AM, Soufi N, Chambers KT, Chen Z, Schweitzer GG, McCommis KS, Erion DM, Graham MJ, Su X, Finck BN (2014) Abrogating monoacylglycerol acyltransferase activity in liver improves glucose tolerance and hepatic insulin signaling in obese mice. Diabetes 637:2284–2296. https://doi.org/10.2337/db13-1502
Watt MJ (2009) Storing up trouble: does accumulation of intramyocellular triglyceride protect skeletal muscle from insulin resistance? Clin Exp Pharmacol Physiol 361:5–11. https://doi.org/10.1111/j.1440-1681.2008.05075.x
Acknowledgements
We thank our lab members for critical reading of the manuscript and helpful discussions.
Funding
This work was supported by Lingnan Modern Agriculture Project (NT2021005), the National Key R & D Program of China (2020YFA0509500 and 2021YFA0805903), National Natural Science Fundation for Distinguished Young Scholars (32025034 and 31925036) and the Agricultural Science and Technology Innovation Program (ASTIP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The project was designed by YW, JZ and NY. Material preparation, data collection and analysis were performed by YZ, CC, JP, SY, TW and CT. Lipid measurement were performed by GS and SML. The first draft of the manuscript was written by YZ and YW, and all authors commented on previous versions of manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was performed in line with the principles of Animal Research Panel of the Committee on Research Practice. Approval was granted by Animal Ethics Committee of the Institute of Animal Science (No. IOZ20190077).
Consent for publication
Human experiments are not involved in this article.
Consent to participate
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Chen, C., Pan, J. et al. Adipocyte Rnf20 ablation increases the fast-twitch fibers of skeletal muscle via lysophosphatidylcholine 16:0. Cell. Mol. Life Sci. 80, 243 (2023). https://doi.org/10.1007/s00018-023-04896-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-023-04896-4