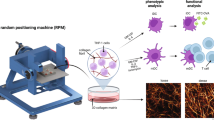
Abstract
Human space travel and exploration are of interest to both the industrial and scientific community. However, there are many adverse effects of spaceflight on human physiology. In particular, there is a lack of understanding of the extent to which microgravity affects the immune system. T cells, key players of the adaptive immune system and long-term immunity, are present not only in blood circulation but also reside within the tissue. As of yet, studies investigating the effects of microgravity on T cells are limited to peripheral blood or traditional 2D cell culture that recapitulates circulating blood. To better mimic interstitial tissue, 3D cell culture has been well established for physiologically and pathologically relevant models. In this work, we utilize 2D cell culture and 3D collagen matrices to gain an understanding of how simulated microgravity, using a random positioning machine, affects both circulating and tissue-resident T cells. T cells were studied in both resting and activated stages. We found that 3D cell culture attenuates the effects of simulated microgravity on the T cells transcriptome and nuclear irregularities compared to 2D cell culture. Interestingly, simulated microgravity appears to have less effect on activated T cells compared to those in the resting stage. Overall, our work provides novel insights into the effects of simulated microgravity on circulating and tissue-resident T cells which could provide benefits for the health of space travellers.








Similar content being viewed by others
Data availability
The datasets generated and analyzed for this study will be available upon request.
References
Witzel I-I, Nasser R, Garcia-Sabaté A et al (2018) Deconstructing immune microenvironments of lymphoid tissues for reverse engineering. Adv Healthc Mater. https://doi.org/10.1002/adhm.201801126
Zhou H, Li B, Li J et al (2019) Dysregulated T cell activation and aberrant cytokine expression profile in systemic lupus erythematosus. Mediators Inflamm 2019:1–11. https://doi.org/10.1155/2019/8450947
Tores MI, López-Casado MA, León de CP et al (2017) Physiology and pathology of immune dysregulation: regulatory T cells and anergy. In: Rezaei N (ed) Physiology and pathology of immunology. IntechOpen
Fulop T, Larbi A, Wikby A et al (2005) Dysregulation of T-cell function in the elderly. Drugs Aging 22:589–603. https://doi.org/10.2165/00002512-200522070-00005
Blaber E, Marçal H, Burns BP (2010) Bioastronautics: the influence of microgravity on astronaut health. Astrobiology 10:463–473. https://doi.org/10.1089/ast.2009.0415
ElGindi M, Sapudom J, Ibrahim IH et al (2021) May the force be with you (Or Not): the immune system under microgravity. Cells 10:1941. https://doi.org/10.3390/cells10081941
Ferranti F, Del BM, Pacelli C (2021) Advantages and limitations of current microgravity platforms for space biology research. Appl Sci 11:1–18. https://doi.org/10.3390/app11010068
Oluwafemi FA, Neduncheran A (2022) Analog and simulated microgravity platforms for life sciences research: Their individual capacities, benefits and limitations. Adv Sp Res 69:2921–2929. https://doi.org/10.1016/j.asr.2022.01.007
Herranz R, Anken R, Boonstra J et al (2013) Ground-based facilities for simulation of microgravity: organism-specific recommendations for their use, and recommended terminology. Astrobiology 13:1–17. https://doi.org/10.1089/ast.2012.0876
Bradbury P, Wu H, Choi JU et al (2020) Modeling the impact of microgravity at the cellular level: implications for human disease. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2020.00096
Iwase S, Nishimura N, Tanaka K, Mano T (2020) Effects of microgravity on human physiology. In: Beyond LEO (ed) Human health issues for deep space exploration [Working Title]. IntechOpen
Sonnenfeld G (2002) The immune system in space. Stress Int J Biol Stress 34:2021–2027. https://doi.org/10.1249/01.MSS.0000039073.04569.B5
Akiyama T, Horie K, Hinoi E et al (2020) How does spaceflight affect the acquired immune system? npj Microgravity 6:1–7. https://doi.org/10.1038/s41526-020-0104-1
Morabito C, Lanuti P, Caprara GA et al (2019) Physiological responses of jurkat lymphocytes to simulated microgravity conditions. Int J Mol Sci 20:1892. https://doi.org/10.3390/ijms20081892
Kumari R, Singh KP, DuMond JW (2009) Simulated microgravity decreases DNA repair capacity and induces DNA damage in human lymphocytes. J Cell Biochem 107:723–731. https://doi.org/10.1002/jcb.22171
Singh KP, Kumari R, DuMond JW (2010) Simulated microgravity-induced epigenetic changes in human lymphocytes. J Cell Biochem 111:123–129. https://doi.org/10.1002/jcb.22674
Vahlensieck C, Thiel CS, Zhang Y et al (2021) Gravitational force—induced 3D chromosomal conformational changes are associated with rapid transcriptional response in human T cells. Int J Mol Sci 22:9426. https://doi.org/10.3390/ijms22179426
Thiel CS, Hauschild S, Huge A et al (2017) Dynamic gene expression response to altered gravity in human T cells. Sci Rep 7:1–22. https://doi.org/10.1038/s41598-017-05580-x
Sapudom J, Pompe T (2018) Biomimetic tumor microenvironments based on collagen matrices. Biomater Sci 6:2009–2024. https://doi.org/10.1039/C8BM00303C
Sapudom J, Rubner S, Martin S et al (2015) The phenotype of cancer cell invasion controlled by fibril diameter and pore size of 3D collagen networks. Biomaterials 52:367–375. https://doi.org/10.1016/j.biomaterials.2015.02.022
Franke K, Sapudom J, Kalbitzer L et al (2014) Topologically defined composites of collagen types I and V as in vitro cell culture scaffolds. Acta Biomater 10:2693–2702. https://doi.org/10.1016/j.actbio.2014.02.036
Brignall R, Cauchy P, Bevington SL et al (2017) Integration of kinase and calcium signaling at the level of chromatin underlies inducible gene activation in T cells. J Immunol 199:2652–2667. https://doi.org/10.4049/jimmunol.1602033
ElGindi M, Ibrahim IH, Sapudom J et al (2021) Engineered microvessel for cell culture in simulated microgravity. Int J Mol Sci 22:6331. https://doi.org/10.3390/ijms22126331
Borst AG, van Loon JJWA (2009) Technology and developments for the random positioning machine, RPM. Microgravity Sci Technol 21:287–292. https://doi.org/10.1007/s12217-008-9043-2
Yen J-C, Chang F-J, Chang S (1995) A new criterion for automatic multilevel thresholding. IEEE Trans Image Process 4:370–378. https://doi.org/10.1109/83.366472
Sapudom J, Karaman S, Mohamed WKE et al (2021) 3D in vitro M2 macrophage model to mimic modulation of tissue repair. npj Regen Med 6:83. https://doi.org/10.1038/s41536-021-00193-5
Andrews S (2010) FastQC: a quality control tool for high throughput sequence data
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Chen S, Zhou Y, Chen Y, Gu J (2018) fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34:i884–i890. https://doi.org/10.1093/bioinformatics/bty560
Kim D, Langmead B, Salzberg SL (2015) HISAT: a fast spliced aligner with low memory requirements. Nat Methods 12:357–360. https://doi.org/10.1038/nmeth.3317
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25:2078–2079. https://doi.org/10.1093/bioinformatics/btp352
Anders S, Pyl PT, Huber W (2015) HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 31:166–169. https://doi.org/10.1093/bioinformatics/btu638
Pertea M, Kim D, Pertea GM et al (2016) Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat Protoc 11:1650–1667. https://doi.org/10.1038/nprot.2016.095
García-Alcalde F, Okonechnikov K, Carbonell J et al (2012) Qualimap: evaluating next-generation sequencing alignment data. Bioinformatics 28:2678–2679. https://doi.org/10.1093/bioinformatics/bts503
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Ge SX, Son EW, Yao R (2018) iDEP: an integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinformatics 19:534. https://doi.org/10.1186/s12859-018-2486-6
Kim SC, Yu D, Cho SB (2018) COEX-seq: convert a variety of measurements of gene expression in RNA-Seq. Genomics Inform 16:e36. https://doi.org/10.5808/GI.2018.16.4.e36
Krämer A, Green J, Pollard J, Tugendreich S (2014) Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 30:523–530. https://doi.org/10.1093/bioinformatics/btt703
Grillo A, Carfagnay M, Federicoz S (2014) The Darcy-Forchheimer law for modelling fluid flow in biological tissues. Theor Appl Mech 41:283–322. https://doi.org/10.2298/TAM1404281G
Wuest SL, Stern P, Casartelli E, Egli M (2017) Fluid dynamics appearing during simulated microgravity using random positioning machines. PLoS ONE 12:e0170826. https://doi.org/10.1371/journal.pone.0170826
Geuzaine C, Remacle J-F (2020) Gmsh. Retrieved from http://gmsh.info/
Weissgerber TL, Garcia-Valencia O, Garovic VD et al (2018) Meta-research: why we need to report more than “Data were Analyzed by t-tests or ANOVA.” Elife 7:e36163. https://doi.org/10.7554/eLife.36163
Paulsen K, Thiel C, Timm J et al (2010) Microgravity-induced alterations in signal transduction in cells of the immune system. Acta Astronaut 67:1116–1125. https://doi.org/10.1016/j.actaastro.2010.06.053
Llanos PJ, Andrijauskaite K, Duraisamy VV et al (2021) Microgravity effect on murine T cells exposed to suborbital flight aboard Blue Origin’s New Shepard vehicle. biorxiv. https://doi.org/10.1101/2021.05.13.443970
Crucian BE, Cubbage ML, Sams CF (2000) Altered cytokine production by specific human peripheral blood cell subsets immediately following space flight. J Interf Cytokine Res 20:547–556. https://doi.org/10.1089/10799900050044741
Ullm F, Pompe T (2021) Fibrillar biopolymer-based scaffolds to study macrophage-fibroblast crosstalk in wound repair. Biol Chem 402:1309–1324. https://doi.org/10.1515/hsz-2021-0164
Alatoom A, Sapudom J, Soni P et al (2020) Artificial biosystem for modulation of interactions between antigen-presenting cells and T cells. Adv Biosyst 4:2000039. https://doi.org/10.1002/adbi.202000039
Tai Y, Wang Q, Korner H et al (2018) Molecular mechanisms of T cells activation by dendritic cells in autoimmune diseases. Front Pharmacol. https://doi.org/10.3389/fphar.2018.00642
Tauber S, Hauschild S, Paulsen K et al (2015) Signal transduction in primary human T lymphocytes in altered gravity during parabolic flight and clinostat experiments. Cell Physiol Biochem 35:1034–1051. https://doi.org/10.1159/000373930
Ai W, Li H, Song N et al (2013) Optimal method to stimulate cytokine production and its use in immunotoxicity assessment. Int J Environ Res Public Health 10:3834–3842. https://doi.org/10.3390/ijerph10093834
Shi R, Re D, Dudl E et al (2010) Chemical biology strategy reveals pathway-selective inhibitor of NF-κB activation induced by protein kinase C. ACS Chem Biol 5:287–299. https://doi.org/10.1021/cb9003089
Hellweg CE, Arenz A, Bogner S et al (2006) Activation of nuclear factor κB by different agents. Ann N Y Acad Sci 1091:191–204. https://doi.org/10.1196/annals.1378.066
Lin H-P, Chang J-Y, Lin S-R et al (2011) Identification of an in vivo MEK/WOX1 complex as a master switch for apoptosis in T cell leukemia. Genes Cancer 2:550–562. https://doi.org/10.1177/1947601911418498
Mandl JN, Liou R, Klauschen F et al (2012) Quantification of lymph node transit times reveals differences in antigen surveillance strategies of naïve CD4 + and CD8 + T cells. Proc Natl Acad Sci 109:18036–18041. https://doi.org/10.1073/pnas.1211717109
Cibrián D, Sánchez-Madrid F (2017) CD69: from activation marker to metabolic gatekeeper. Eur J Immunol 47:946–953. https://doi.org/10.1002/eji.201646837
Riley JL (2009) PD-1 signaling in primary T cells. Immunol Rev 229:114–125. https://doi.org/10.1111/j.1600-065X.2009.00767.x
Lichtenegger FS, Rothe M, Schnorfeil FM et al (2018) Targeting LAG-3 and PD-1 to enhance T cell activation by antigen-presenting cells. Front Immunol. https://doi.org/10.3389/fimmu.2018.00385
Chikuma S, Terawaki S, Hayashi T et al (2009) PD-1-Mediated Suppression of IL-2 Production Induces CD8 + T Cell Anergy In Vivo. J Immunol 182:6682–6689. https://doi.org/10.4049/jimmunol.0900080
Yang Z-Z, Kim HJ, Villasboas JC et al (2017) Expression of LAG-3 defines exhaustion of intratumoral PD-1+ T cells and correlates with poor outcome in follicular lymphoma. Oncotarget 8:61425–61439. https://doi.org/10.18632/oncotarget.18251
Low EK, Brudvik E, Kuhlman B et al (2018) Microgravity impairs DNA damage repair in human hematopoietic stem/progenitor cells and inhibits their differentiation into dendritic cells. Stem Cells Dev 27:1257–1267. https://doi.org/10.1089/scd.2018.0052
Gao Y, Xu D, Zhao L, Sun Y (2017) The DNA damage response of C. elegans affected by gravity sensing and radiosensitivity during the Shenzhou-8 spaceflight. Mutat Res Mol Mech Mutagen 795:15–26. https://doi.org/10.1016/j.mrfmmm.2017.01.001
Singh R, Rajput M, Singh RP (2021) Simulated microgravity triggers DNA damage and mitochondria-mediated apoptosis through ROS generation in human promyelocytic leukemic cells. Mitochondrion 61:114–124. https://doi.org/10.1016/j.mito.2021.09.006
Moreno-Villanueva M, Wong M, Lu T et al (2017) Interplay of space radiation and microgravity in DNA damage and DNA damage response. npj Microgravity 3:14. https://doi.org/10.1038/s41526-017-0019-7
dos Santos Á, Toseland CP (2021) Regulation of nuclear mechanics and the impact on DNA damage. Int J Mol Sci 22:3178. https://doi.org/10.3390/ijms22063178
Hart M, Adams SD, Draviam VM (2021) Multinucleation associated DNA damage blocks proliferation in p53-compromised cells. Commun Biol 4:451. https://doi.org/10.1038/s42003-021-01979-5
Difilippantonio S, Gapud E, Wong N et al (2008) 53BP1 facilitates long-range DNA end-joining during V(D)J recombination. Nature 456:529–533. https://doi.org/10.1038/nature07476
Groom JR, Luster AD (2011) CXCR3 in T cell function. Exp Cell Res 317:620–631. https://doi.org/10.1016/j.yexcr.2010.12.017
Wellmann S, Truss M, Bruder E et al (2010) The RNA-binding protein RBM3 is required for cell proliferation and protects against serum deprivation-induced cell death. Pediatr Res 67:35–41. https://doi.org/10.1203/PDR.0b013e3181c13326
Louis F, Deroanne C, Nusgens B et al (2015) RhoGTPases as key players in mammalian cell adaptation to microgravity. Biomed Res Int 2015:1–17. https://doi.org/10.1155/2015/747693
Bros H, Moll G (2019) RhoA as a key regulator of innate and adaptive immunity. Cells 8:733. https://doi.org/10.3390/cells8070733
White RR, Vijg J (2016) Do DNA double-strand breaks drive aging? Mol Cell 63:729–738. https://doi.org/10.1016/j.molcel.2016.08.004
Princz A, Tavernarakis N (2017) The role of SUMOylation in ageing and senescent decline. Mech Ageing Dev 162:85–90. https://doi.org/10.1016/j.mad.2017.01.002
Dinarelli S, Longo G, Dietler G et al (2018) Erythrocyte’s aging in microgravity highlights how environmental stimuli shape metabolism and morphology. Sci Rep 8:5277. https://doi.org/10.1038/s41598-018-22870-0
Takahashi H, Nakamura A, Shimizu T (2021) Simulated microgravity accelerates aging of human skeletal muscle myoblasts at the single cell level. Biochem Biophys Res Commun 578:115–121. https://doi.org/10.1016/j.bbrc.2021.09.037
Wang J, Zhang J, Bai S et al (2009) Simulated microgravity promotes cellular senescence via oxidant stress in rat PC12 cells. Neurochem Int 55:710–716. https://doi.org/10.1016/j.neuint.2009.07.002
Li X, Liu L, Yang S et al (2014) Histone demethylase KDM5B is a key regulator of genome stability. Proc Natl Acad Sci 111:7096–7101. https://doi.org/10.1073/pnas.1324036111
Wu L, Cao J, Cai WL et al (2018) KDM5 histone demethylases repress immune response via suppression of STING. PLOS Biol 16:e2006134. https://doi.org/10.1371/journal.pbio.2006134
Ehlén Å, Brennan DJ, Nodin B et al (2010) Expression of the RNA-binding protein RBM3 is associated with a favourable prognosis and cisplatin sensitivity in epithelial ovarian cancer. J Transl Med 8:78. https://doi.org/10.1186/1479-5876-8-78
Green AM, Weitzman MD (2019) The spectrum of APOBEC3 activity: from anti-viral agents to anti-cancer opportunities. DNA Repair (Amst) 83:102700. https://doi.org/10.1016/j.dnarep.2019.102700
Rabin RL, Alston MA, Sircus JC et al (2003) CXCR3 is induced early on the pathway of CD4 + T cell differentiation and bridges central and peripheral functions. J Immunol 171:2812–2824. https://doi.org/10.4049/jimmunol.171.6.2812
Masuda K, Ripley B, Nyati KK et al (2016) Arid5a regulates naive CD4+ T cell fate through selective stabilization of Stat3 mRNA. J Exp Med 213:605–619. https://doi.org/10.1084/jem.20151289
Tubau-Juni N, Hontecillas R, Leber A et al (2021) First-in-class topical therapeutic omilancor ameliorates disease severity and inflammation through activation of LANCL2 pathway in psoriasis. Sci Rep 11:19827. https://doi.org/10.1038/s41598-021-99349-y
Leber A, Bassaganya-Riera J, Tubau-Juni N et al (2017) Lanthionine synthetase C-like 2 modulates immune responses to influenza virus infection. Front Immunol. https://doi.org/10.3389/fimmu.2017.00178
Arora R, Lee Y, Wischnewski H et al (2014) RNaseH1 regulates TERRA-telomeric DNA hybrids and telomere maintenance in ALT tumour cells. Nat Commun 5:5220. https://doi.org/10.1038/ncomms6220
Ferrari F, Solari A, Battaglia C, Bicciato S (2011) PREDA: an R-package to identify regional variations in genomic data. Bioinformatics 27:2446–2447. https://doi.org/10.1093/bioinformatics/btr404
Jung P, Zhou X, Iden S et al (2021) T cell stiffness is enhanced upon formation of immunological synapse. Elife. https://doi.org/10.7554/eLife.66643
Makhija E, Jokhun DS, Shivashankar GV (2016) Nuclear deformability and telomere dynamics are regulated by cell geometric constraints. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.1513189113
Shanti A, Samara B, Abdullah A et al (2020) Multi-compartment 3D-cultured organ-on-a-chip: towards a biomimetic lymph node for drug development. Pharmaceutics 12:464. https://doi.org/10.3390/pharmaceutics12050464
Hope JM, Dombroski JA, Pereles RS et al (2022) Fluid shear stress enhances T cell activation through Piezo1. BMC Biol 20:61. https://doi.org/10.1186/s12915-022-01266-7
Choi DH, Jeon B, Lim MH et al (2021) 3D cell culture using a clinostat reproduces microgravity-induced skin changes. npj Microgravity 7:20. https://doi.org/10.1038/s41526-021-00148-6
Sapudom J, ElGindi M, Arnoux M et al (2021) Fibroblast differentiation and matrix remodeling impaired under simulated microgravity in 3D cell culture model. Int J Mol Sci 22:11911. https://doi.org/10.3390/ijms222111911
Hauslage J, Cevik V, Hemmersbach R (2017) Pyrocystis noctiluca represents an excellent bioassay for shear forces induced in ground-based microgravity simulators (clinostat and random positioning machine). npj Microgravity 3:12. https://doi.org/10.1038/s41526-017-0016-x
Acknowledgements
The authors would like to acknowledge support from NYUAD core technology platform (cell and molecular biology, optical imaging, and bioinformatics, and sequencing).
Funding
The authors acknowledge the support from New York University Abu Dhabi (NYUAD) Faculty Research Fund (AD266) and NYUAD Research Enhancement Fund (RE267).
Author information
Authors and Affiliations
Contributions
Conceptualization, ME, JS, AG-S, and JCT; formal analysis, ME, JS, PL; funding acquisition, JCT; investigation, ME and JS; methodology, ME and JS; supervision, JS, MD and JCT; visualization, ME, JS, and AG-S; writing—original draft, ME; writing—review and editing, JS, AG-S, and JCT. All authors have read and agreed to the published version of the manuscript
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
ElGindi, M., Sapudom, J., Laws, P. et al. 3D microenvironment attenuates simulated microgravity-mediated changes in T cell transcriptome. Cell. Mol. Life Sci. 79, 508 (2022). https://doi.org/10.1007/s00018-022-04531-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00018-022-04531-8